Disease of the Month – Amyotrophic Lateral Sclerosis (ALS)
Shots:
- To keep our readers acquainted with several disease conditions, ongoing trials, and available treatment options, PharmaShots brings every month a detailed take on a particular disease after thorough research
- To continue the series for the disease of the month, PharmaShots brings this month a summary of the disease Amyotrophic Lateral Sclerosis (ALS), a rare neurological disease that affects motor neurons
- May is observed as ALS Awareness Month to raise awareness for ALS, a disease that affects people in every country around the globe
Introduction[1]
Amyotrophic lateral sclerosis (ALS), aka Lou Gehrig’s disease, is a rare neurological condition that affects nerve cells in the brain and spinal cord that control voluntary muscle movement. It causes muscle twitching and weakness in an arm or leg, trouble swallowing, or slurred speech. The symptoms get worse over time and the disease has no cure or no effective treatment to reverse its progression.
As the disease progresses, muscles get weak, and atrophy spread to other parts of the body, which may develop problems like:
- People with ALS will not be able to stand, walk or use their hands and arms
- Chewing food and swallowing (dysphagia)
- Speaking or forming words (dysarthria)
- Individuals eventually lose the ability to breathe on their own and must depend on a ventilator
- Maintaining weight and malnourishment
- Muscle cramps and neuropathy (nerve damage or disease)
- Anxiety and depression, as people with ALS lose their function to reason, remember, understand, and are unaware of their progressive loss of function
- People experience problems with language or decision-making
- Develop a form of dementia over time
ALS doesn’t affect the ability to taste, touch, smell, or hear. Most people with ALS die from respiratory failure, usually within 3-5 years from when the symptoms first appear
Causes:
ALS is inherited in 5% to 10% of people. For the rest, the cause is still unknown
However, risk factors may include:
- Heredity – 5-10% of the people with ALS are inherited (familial ALS). Children have a 50% chance of developing the disease
- Age – ALS is common between the ages of 40 and the mid-60s
- Sex – Before, the age of 65, more men than women develop ALS. Sex difference disappears after 70 years
- Genetics – Similarities were found in the genetic variations of people with familial ALS and people with non-inherited ALS. These genetic variations might make people more susceptible to ALS
- Smoking – Greatest for women, particularly after menopause
- Environmental toxin exposure – Exposure to lead or other substances in the workplace or at home might be linked to ALS. According to studies, no single agent or chemical is associated with ALS
Symptoms[2]
- Difficulty walking or doing normal daily activities
- Tripping and falling
- Weakness in legs, feet, or ankles
- Hand weakness or clumsiness
- Slurred speech or trouble swallowing
- Muscle cramps and twitching in arms, shoulders, and tongue
- Inappropriate crying, laughing, or yawning
- Cognitive and behavioral changes
Diagnosis[3]
- Electrodiagnostic tests, including electromyography (EMG) and nerve conduction velocity (NCV) to detect the electrical activity of muscle fibers
- Blood and urine studies, including high-resolution serum protein electrophoresis, thyroid and parathyroid hormone levels, and 24-hour urine collection for heavy metals
- Spinal tap
- X-rays, including MRI
- Myelogram of the cervical spine
- Muscle and/or nerve biopsy
- A thorough neurological examination
Epidemiology[4]
The incidence of ALS is ~1–2.6 cases per 100,000 persons annually, whereas the prevalence is ~6 cases per 100,000. ALS is 20% more common in men than women.
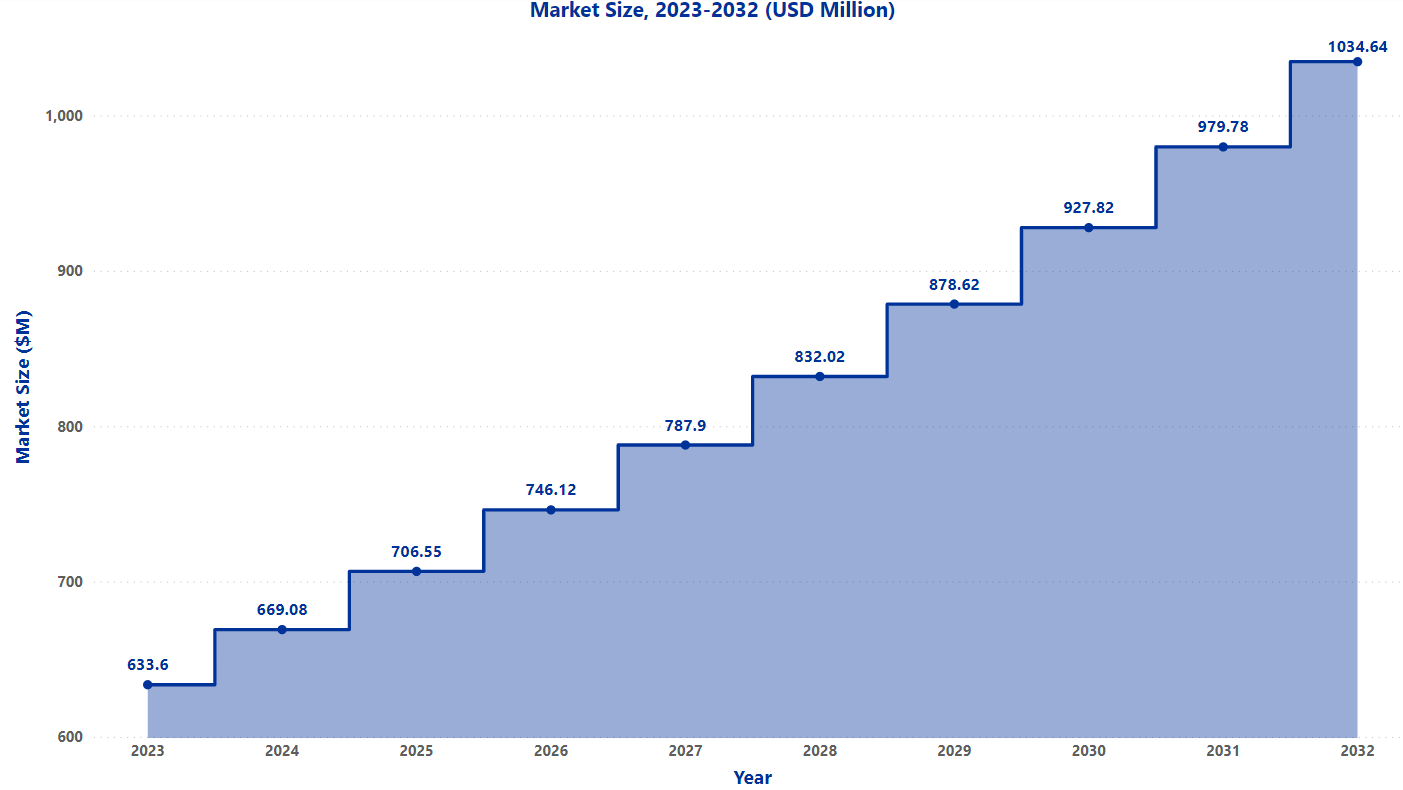
Market Size[5]
The global ALS treatment market was valued at $600M in 2022 and is expected to increase by $1034M in 2032 at a CAGR of 5.6%
Treatment
- Medications: Currently, there is no cure available to reverse damaged motor neurons or cure ALS. However, available medications can reduce the progression of the disease and prevent complications by improving the patient’s survival and quality of life
- Physical therapy, nutritional support, and breathing care can help in improving overall health, and those having difficulty speaking can benefit from devices such as computer-based speech synthesizers
- Rehabilitation Therapy
- Speech Therapy
- Assistive devices such as splints, braces and grab bars, a brain-computer interface along with special equipment including wheelchairs and electric beds can assist in communicating and living independently. More efficient, mobile, and auditory-based BCIs are being developed for those with severe paralysis or visual impairments
Key Players in the Market[6]
Riluzole is the first medication used to reduce damage to motor neurons by decreasing levels of glutamate. Initially, it was approved in 1996 as an oral tablet (Rilutek). It is also available in other formulations to prolong survival by a few months. The thickened liquid form (Tiglutik) or the tablet (Exservan)
Edaravone (Radicava) is administered intravenously and has been shown to slow the decline in the clinical assessment of daily functioning in people with ALS.
In April 2023, the US FDA granted accelerated approval to Qalsody (tofersen) (100mg/15mL) being developed by Biogen for the treatment of adults with ALS who have a mutation in the SOD1 gene
Other key leaders with approved molecules in the market are: Amylyx, ITF Pharma etc.

Clinical Trial Analysis[7]
As of May 05, 2023, a total of 94 clinical trials are going on worldwide for ALS. Some of the key molecules involved in the trials are BIIB 067 (Biogen) and Masitinib (AB Sciences), SLS-005 (Seelos Therapeutics),etc.
Based on G10 geography distribution, the interventional clinical trials are classified in the below-mentioned graph in two groups based on their status i.e., active (recruiting, active, not recruiting, not yet recruiting and enrolling by invitation, suspended) and inactive (withdrawn, terminated and trials with unknown status). The interpretation showed that the highest no. of trials being conducted in the USA and the least number of trials are reported in China (as represented in the graph)


Recent Advancements in ALS
- Researchers are identifying additional genes that may cause or put a person at risk for either familial or sporadic ALS and are involved in discovering new genes. By using novel gene editing tools, researchers are now able to rapidly identify new genes in the human genome involved in ALS. Additionally, researchers are looking at the potential role of epigenetics in ALS development.
- Development of biomarkers is underway.
- Studies of drug-like compounds, gene therapy approaches, antibodies, and cell-based therapies in a range of disease models are ongoing.
- NINDS-funded scientists are using stem cells to grow human spinal cord sections on tissue chips to help better understand the function of neurons involved in ALS.
Reference:
1. NIH
3. Mayo clinic
6. ALS.org
For Deep dive landscape please mail us at connect@pharmshots.com
Related Post: Disease of the Month: Hemophilia



