CASI Pharmaceuticals and BioInvent Reveal P-I Study Results of BI-1206 for the Treatment of Non-Hodgkin’s Lymphoma in China
Shots:
- CASI (BI-1206’s licensee in China, Hong Kong, Macau & Taiwan) & BioInvent have reported P-I dose-escalation study results investigating the safety, tolerability, pharmacology & clinical activity of BI-1206 (IV) + rituximab for treating r/r indolent Non-Hodgkin’s Lymphoma (iNHL)
- The results demonstrated, among 8 evaluable patients, 4 of them experienced PR while 1 patient with relapsed marginal zone lymphoma (MZL) experienced CR & maintained a durable complete remission for >20 wks.
- The company’s BI-1206 is currently under evaluation in 3 P-I/II trials across the US, the EU, Brazil & China. 2 of them are assessing BI-1206 + rituximab for iNHL incl. FL, MCL & MZL while the 3rd one is investigating BI-1206 + Keytruda for solid tumors
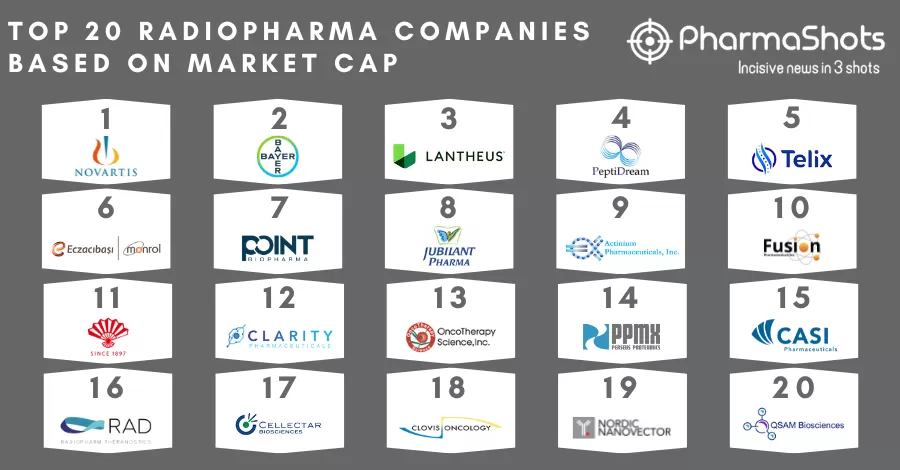
Ref: CASI Pharmaceuticals | Image: CASI Pharmaceuticals
Related News:- BioInvent Signs a Clinical Supply Agreement with AstraZeneca to Assess BI-1206 for Treating Non-Hodgkin's Lymphoma
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.