Disease of the Month: Epilepsy
INTRODUCTION
Also known as seizure disorder, epilepsy is a non-communicable chronic disorder of the brain and is the fourth most common neurological disease globally. It is a central nervous system (CNS) disorder characterized by recurrent episodes of seizures that are short changes in normal brain activity which led to brief incidences of involuntary movements either involving only a part of the body or the whole body. The seizures are sometimes accompanied by loss of bowel or bladder control or consciousness. An individual is diagnosed with epilepsy when they have had two or more episodes of seizures. One seizure does not necessarily indicate epilepsy since up to 10% of people worldwide experience one seizure over their lifetime. Written records of epilepsy date as far back as 4000BC, making it one of the world’s oldest recognized medical conditions.
The disease affects approximately 50 million people around the globe. It affects both males and females irrespective of their age, race, and ethnic background. Through our analysis, we deciphered that due to the maximum of clinical trials being carried out in the EU5 region, the number of Epileptic patients can be expected to be more in the EU5/Europe with China on the other hand conducting the least number of trials or they have reported lesser trials in epilepsy (as represented in the graph).
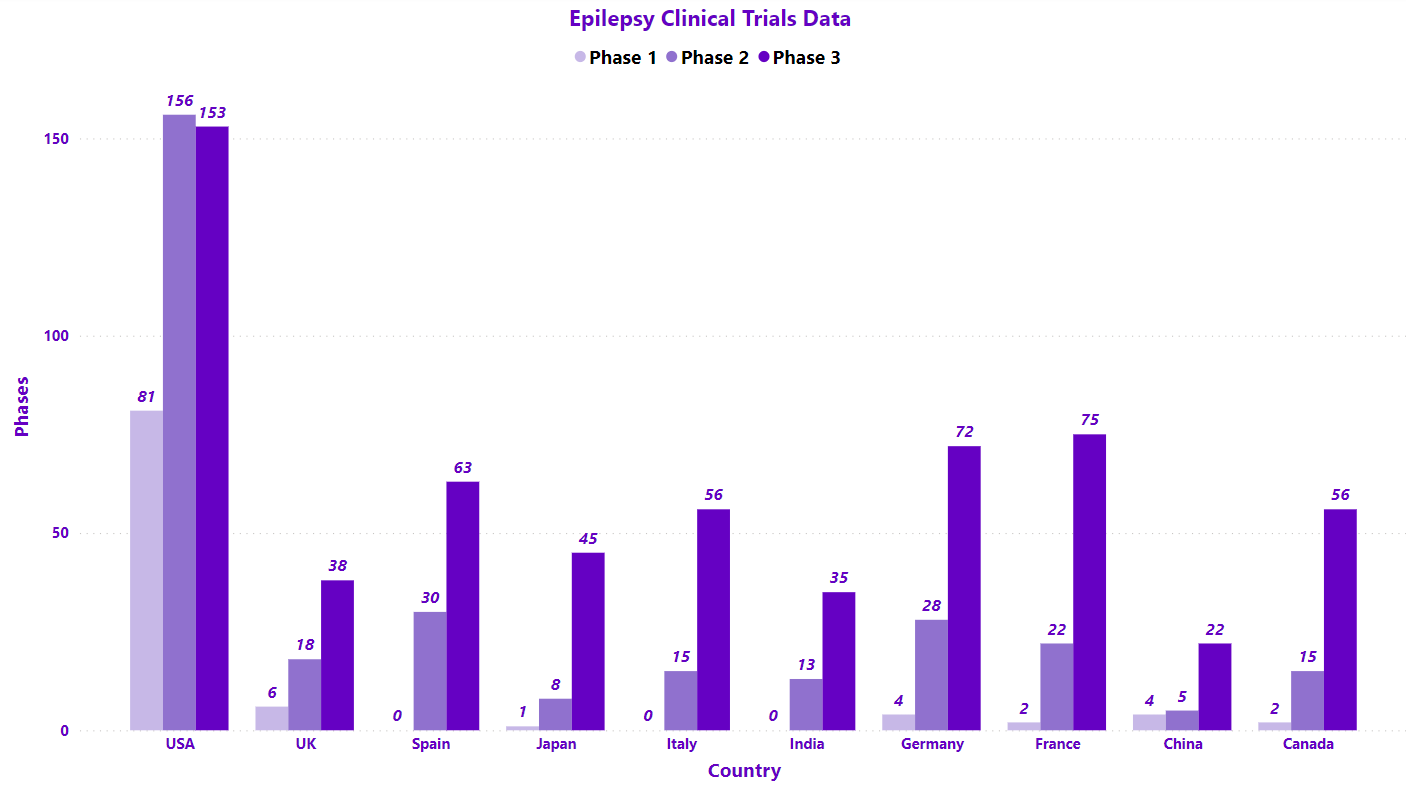
*The graphical interpretation is based on our point of view of the clinical data
CAUSES
Epilepsy is not a contagious disease. Though various underlying diseases can cause epilepsy, in roughly 50% of cases around the world the disease’s origin is still unknown. Some of the causes include:
- Severe head injury
- Some genetic syndromes
- A stroke that restricts amount of oxygen to the brain
- A brain tumor
- Congenital abnormalities associated with brain malformations
- Some brain infections, like neurocysticercosis, meningitis, or encephalitis
- Brain damage from perinatal or prenatal causes
TYPE OF SEIZURES
Seizures are caused due to excessive electrical discharge among the group of brain cells. Such discharges can occur in different parts of the brain. Doctors classify epileptic seizures into two types depending on the area and manner of initiation of the abnormal brain activity:
1. Focal Seizures- these seizures are also called partial seizures and occur due to abnormal activity in only one area of the brain. They are further categorized into two types:
- Focal seizures without consciousness loss- they were also referred to as simple partial seizures. This kind may cause change or alteration of emotions, and the way things smell, feel, look, sound or taste without any loss of consciousness. Individuals may experience déjà vu. It may also cause involuntary jerking of one body part and sudden sensory symptoms like dizziness, flashing lights, and tingling.
- Focal seizures with impaired awareness- once they were also called complex partial seizures. This kind of seizure may appear to be like a dream involving a loss or change of awareness or consciousness. Experiencing this seizure, the patient may not respond to surroundings normally or keep performing repetitive movements like chewing, hand rubbing, swallowing, or walking in circles.
2. Generalized Seizures- these seizures affect both areas of the brain. They are further categorized into 6 types:
- Absence Seizures- also known as petit mal seizures and occur typically in children. It lasts for only 5-10 seconds and happens as often as 100 times a day. They cause a brief loss of awareness and are characterized by staring into space with or without subtle body movements.
- Tonic Seizures- they cause stiffness of the muscles (usually back, arms, and legs muscles). It may cause the person to fall to the ground also affecting the consciousness. The individual usually feels tired after this type of seizure.
- Atonic Seizures- they are also known as drop seizures. It causes a loss of muscle control most often affecting the leg muscles leading to the collapse or falling of the person.
- Clonic Seizures- these seizures usually affect the face, neck, and arms. They are associated with repeated or rhythmic jerking muscle movements.
- Myoclonic Seizures- they usually appear as a sudden twitch or jerk and generally affect arms, upper body, and legs.
- Tonic-clonic Seizures- also known as grand mal seizures, they are the most dramatic kind of epilepsy seizure. They can cause body stiffness, shaking, twitching, and loss of consciousness. They can also cause biting of the tongue or loss of bladder control sometimes.
The patient may experience more than one kind of seizure.
SYMPTOMS
As epilepsy is a result of abnormal brain activity, seizures can affect any process of brain coordination. Symptoms of seizures include:
- Muscles stiffness
- Temporary confusion
- Psychological impacts like anxiety, fear, or déjà vu
- Uncontrolled jerking movements of legs and arms, referred as ‘fit’
- Loss of awareness or consciousness
- Disturbances of sensation, mood, movement, or any cognitive functions
Both physical issues (such as fractures and bruising from seizures-related traumas) and psychological issues, such as anxiety and sadness, are more common in people with epilepsy. Similarly, to this, patients with epilepsy are up to three times more likely to die prematurely than the general population, with rural areas and low- and middle-income nations having the highest rates of early mortality.
The type of seizure determines the specific symptoms. The symptoms will be consistent from episode to episode since, in the majority of situations, a person with epilepsy tends to experience the same sort of seizure every time.
TREATMENT
There is no permanent cure available to treat epilepsy, but seizures can be controlled. The first step toward the treatment is to select the appropriate AED (Anti-Epileptic Drug). Approximately 70% of epileptic patients can get rid of their seizures by using suitable antiseizure medicines. The use of AEDs can be discontinued only if the individual does not experience seizures for 2 years also considering relevant personal, clinical and social factors. Seizures stopping medicine should not be stopped suddenly as it can cause status epilepticus (seizures that will not stop). The recurrence of seizures can be predicted using documented etiology of seizures or an abnormal EEG pattern.
VIMPAT (lacosamide) is one such drug developed by UCB. VIMPAT has been approved by FDA for the treatment of primary generalized tonic-clonic seizures and expanded pediatric use for epileptic patients on 17 November 2020. VIMPAT is available in two forms: as VIMPAT Tablets of multiple doses and sizes and as VIMPAT Oral Solution which comes in strawberry-flavored liquid. Similar to other AED, VIMPAT can also cause suicidal actions or thoughts in a small proportion of people like 1 in 500. It can also cause feelings of dizziness, double vision, coordination or walking problems, or sleepiness. An irregular heartbeat can also occur in patients using VIMPAT.
In case seizures continue to occur, the following treatment options can be considered:
- Dietary Therapy- a special ketogenic diet can be recommended
- Seizure Devices- a small electrical device can be inserted inside the body which will help to control seizures
- Epilepsy Surgery- in cases of persistent uncontrolled seizures, surgery can be performed to remove a small part of the brain responsible for causing seizures
Depending on the individual a person may be able to stop the treatment if seizures disappear or can require treatment throughout their life.
REFERENCES
Related Post: Disease of the Month: Cerebral Palsy | PharmaShots



