
PharmaShots Weekly Snapshots (September 25–29, 2023)
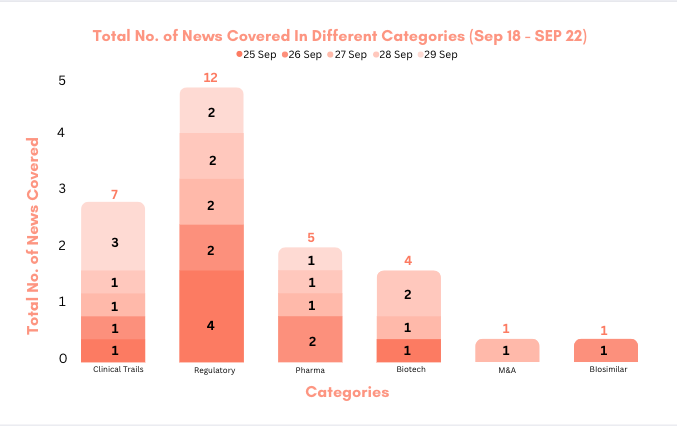
This week PharmaShots’ news was all about the updates on clinical trials, regulatory, biotech, pharma, and M&A. Check out our full report below:

- The US FDA has approved Boehringer Ingelheim and Eli Lilly’s Jardiance (empagliflozin) for adults with chronic kidney disease, based on the P-III trial (EMPA-KIDNEY)
Read more: Boehringer Ingelheim and Eli Lilly
- The NMPA has accepted the NDA of Henlius’ Hansizhuang (serplulimab) for Esophageal Squamous Cell Carcinoma, based on the P-III trial (ASTRUM-007)
Read more: Henlius
- The NMPA has granted the marketing authorization of Sanofi’ Altuviiio for Hemophilia A, based on the positive results incl. the (XTEND-1) trial in adults & adolescents and the (XTEND-Kids) trial in children under 12yrs.
Read more: Sanofi
- The MHLW has approved Genmab’ Epkinly (epcoritamab) for adults patients with Large B-Cell Lymphoma, based on the P-I/II (EPCORE NHL-1 & 3) trials
Read more: Genmab
- The EC has granted conditional marketing authorization for Tepkinly to treat adult patients with r/r DLBCL, based on the results from the P-I/II trial (EPCORE NHL-1)
Read more: AbbVie and Genmab
- The US FDA has accepted the sBLA for Priority Review of Regeneron and Sanofi’ Dupixent to treat Eosinophilic Esophagitis in children aged 1-11Years, based on the P-III (EoE KIDS) trial (Parts A and B)
Read more: Regeneron and Sanofi
- The MHLW has approved UCB’ Rystiggo (rozanolixizumab) and Zilbrysq (zilucoplan) for adult patients with generalized myasthenia gravis in Japan, based on the P-III (MycarinG) & (RAISE) studies
Read more: UCB
- The US FDA has cleared an IND application to initiate a P-II clinical trial of AltruBio’ ALTB-268 for the treatment of ulcerative colitis
Read more: AltruBio
- The US FDA has approved Viatris and Ocuphire’ Ryzumvl (Phentolamine Ophthalmic Solution) for pharmacologically-induced mydriasis, based on the MIRA clinical program
Read more: Viatris and Ocuphire
- Karuna Therapeutics submits the NDA of KarXT to the US FDA for the treatment of Schizophrenia, based on the (EMERGENT) program i.e., (EMERGENT-1/2/3) trials
Read more: Karuna Therapeutics
- The US FDA has approved Amicus Therapeutics’ Pombiliti (cipaglucosidase alfa-atga) + Opfolda for Pompe Disease, based on the P-III study (PROPEL)
Read more: Amicus Therapeutics
- HUTCHMED submits the NDA of Fruquintinib for previously treated metastatic colorectal cancer in Japan, based on the results from the P-III trial (FRESCO-2) as well as results from the P-III (FRESCO) trial
Read more: HUTCHMED

- Novartis highlighted P-III trial (NETTER-2) results of Lutathera as 1L advanced Gastroenteropancreatic Neuroendocrine Tumors demonstrated a significant improvement in PFS over high-dose long-acting octreotide alone
Read more: Novartis
- Lantern Pharma dosed the first patient of LP-184 in the P-I study for advanced solid tumors
Read more: Lantern Pharma
- Ionis highlighted P-III study (Balance) results of Olezarsen for Familial Chylomicronemia Syndrome met its primary efficacy EPs with a reduction in triglyceride levels with olezarsen
Read more: Ionis
- Basilea highlighted P-III study (ERADICATE) results of Ceftobiprole for Staphylococcus Aureus Bacteremia published in the NEJM showed that ceftobiprole was non-inferior to daptomycin with overall treatment success
Read more: Basilea
- Janssen highlighted P-III study (MARIPOSA) results of Rybrevant (amivantamab-vmjw) + Lazertinib for EGFR-mutated NSCLC showed a significant & clinical improvement in PFS
Read more: Janssen
- Ionis Reports P-III Study (NEURO-TTRansform) Results of Eplontersen for Hereditary Transthyretin-Mediated Amyloid Polyneuropathy. In the 66wk. primary analysis, eplontersen showed an improvement across all co-1EPs & 2EPs
Read more: Ionis
Ascletis highlighted positive interim results from the P-IIb expansion cohort of ASC22 (envafolimab) for Chronic Hepatitis B functional cure
Read more: Ascletis

- Pierre Fabre Laboratories & Vernalis to identify pre-clinical candidates against multiple oncology targets
Read more: Pierre Fabre Laboratories & Vernalis
- Ono & Adimab signs an agreement to discover novel antibody drugs for cancer where Ono will get an exclusive rights option to globally develop, manufacture, and commercialize the candidates
Read more: Ono & Adimab
- Ionis & Roche collaborated for two novel RNA-targeted programs for Alzheimer's and Huntington's Disease
Read more: Ionis & Roche
- Ginkgo Bioworks & Pfizer collaborated for RNA-based drug candidates
Read more: Ginkgo Bioworks & Pfizer

- AcuraStem & Takeda collaborated to advance PIKFYVE therapies for amyotrophic lateral sclerosis
Read more: AcuraStem & Takeda
- Valo Health Signs an Agreement with Novo Nordisk to Discover and Develop Novel Treatments for Cardiometabolic Diseases using Valo’s Opal Computational Platform
Read more: Valo Health and Novo Nordisk
- Evommune Collaborated with Maruho to Develop and Commercialize EVO756, a potent, highly selective small molecule antagonist of MRGPRX2 in Japan
Read more: Evommune and Maruho
- Cosmo & Glenmark collaborated for Winlevi in Europe and South Africa. Glenmark will get an exclusive right from Cassiopea, a subsidiary of Cosmo to commercialize Winlevi in 15 EU countries
Read more: Cosmo & Glenmark
- BioCity & AstraZeneca collaborated to evaluate BC3402 + Imfinzi (durvalumab) for Advanced Hepatocellular Carcinoma in China
Read more: BioCity & AstraZeneca

- Alfasigma to buy Intercept Pharmaceuticals for $794M expanding the global footprint in the US market and expanding its portfolio in liver diseases and digestive system disorders
Read more: Alfasigma and Intercept Pharmaceuticals

- The EC has granted marketing authorization for Sandoz’s first and only biosimilar Tyruko to treat relapsing forms of MS
Read more: Sandoz
Related Post: PharmaShots Weekly Snapshots (September 18–22, 2023)
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














