
Top Performing Drug – Ocrevus (July Edition)
Shots:
- In continuation of our previous series on the top-performing drug of the month, based on 2021 revenue, we bring for our readers a concise yet thorough analysis of the drug Ocrevus
- Ocrevus is a prescription medicine used to treat adults with relapsing or primary progressive multiple sclerosis including CIS, RRMS, and SPMS
- PharmaShots presents a detailed take on the key features of Ocrevus with an extensive analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis and informative KOL reviews
Active Ingredient: Ocrelizumab
Dosage Forms & Strengths: Injection: 300 mg/10 mL in a single-dose vial
Mechanism of Action: Antibody-dependent cell cytotoxicity
Originators: Genentech (Roche)
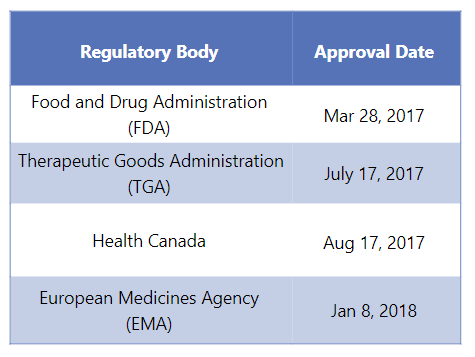
First Approvals: The table below depicts the first approvals of Ocrevus from different regulatory agencies.
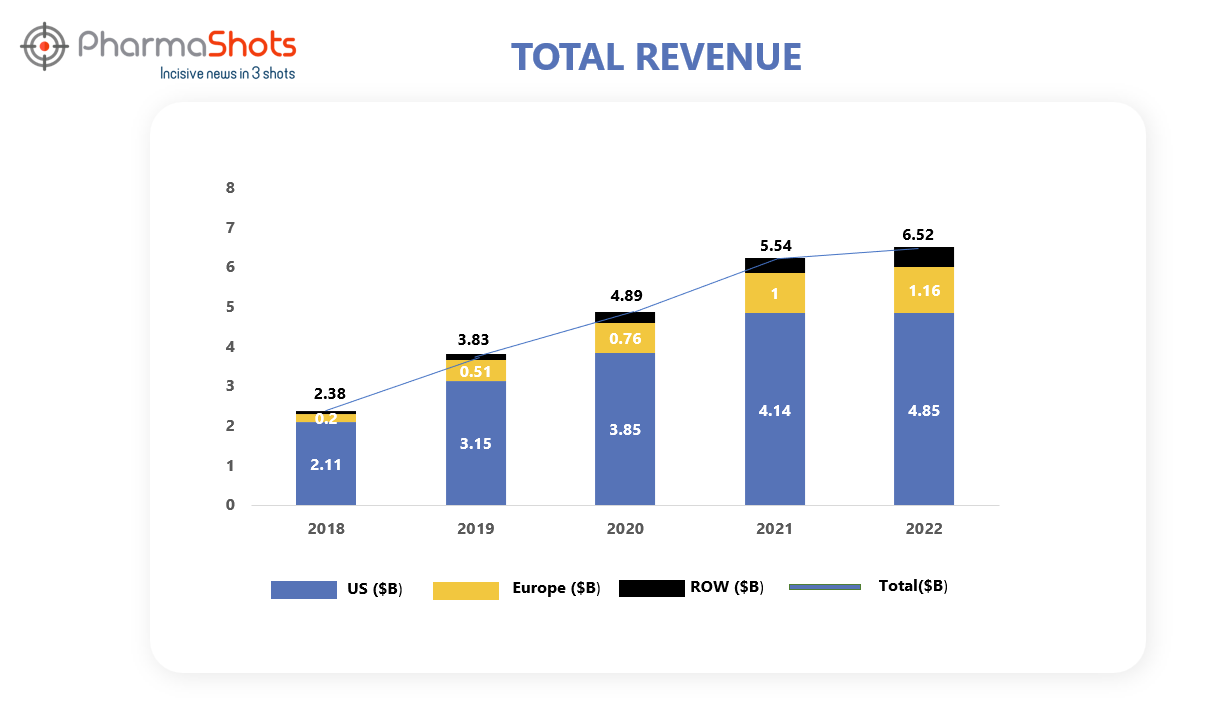
Revenue Analysis1
Ocrevus is developed and commercialized by Roche's subsidiary Genentech. After its approval in 2017 for the treatment of RMS and PPMS, Genentech underwent an agreement with Biogen under which Biogen received a tiered royalty on U.S. net sales from 13.5% and increasing up to 24.0% owing to a condition if the annual net sales exceeded $900.0 million. Moreover, Biogen is entitled to a 3.0% royalty on the net sales of Ocrevus outside the United States. This royalty period extends for 11 years from the initial commercial launch of the product, applicable to each country individually.
In 2022, the demand for Ocrevus remained strong with a sales increase of 17%. The graphical analysis of Ocrevus’ past 5 years’ revenue is showcased below.
 Approved Indications for Ocrevus2
Approved Indications for Ocrevus2
Ocrevus is approved for the treatment of:
- Relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults
- Primary progressive MS, in adults (PPMS)
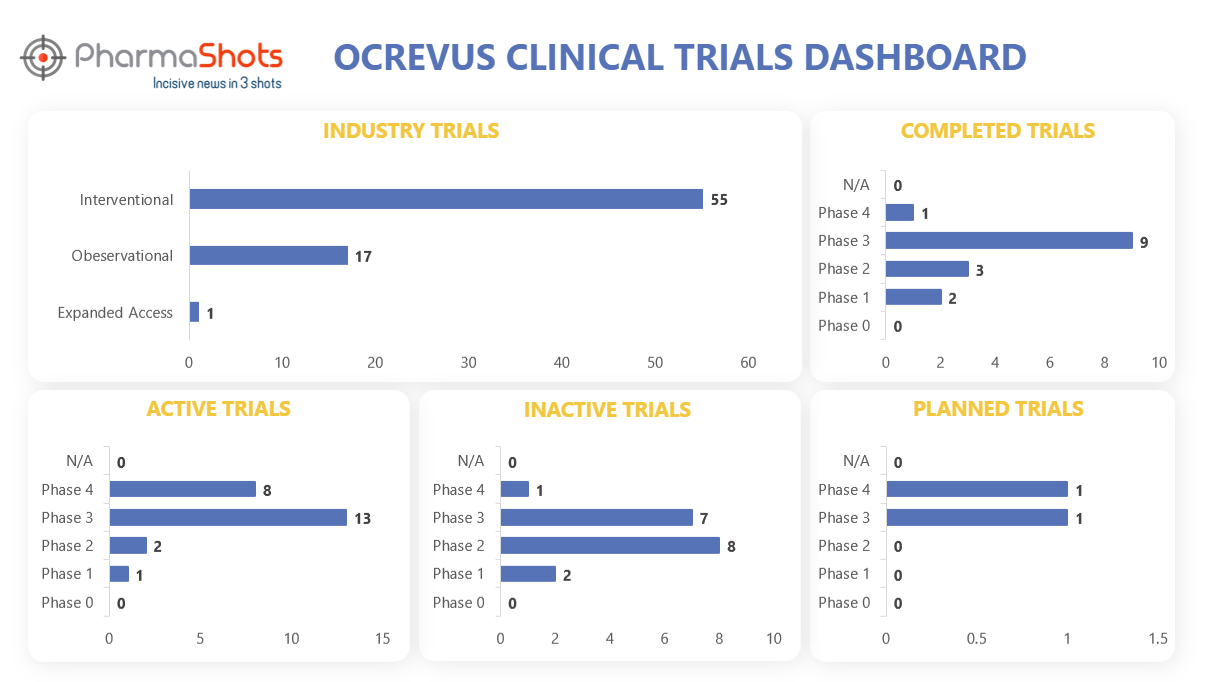
Clinical Trials Analysis3
Ocrevus underwent a total of 118 trials, with 73 of them being industry trials. Among these industry trials, 55 were interventional studies, 17 were observational studies, and 1 was an expanded access trial. (Trials were taken on 5th July 2023)
Ocrevus Trials Representation (Country-wise)4
The stacked column chart below provides a visual representation of the ongoing evaluation of Ocrevus in various indications worldwide. It focuses on the top 10 countries globally and includes only industry trials (interventional studies)
* The chart depicts data till 6th July 2023
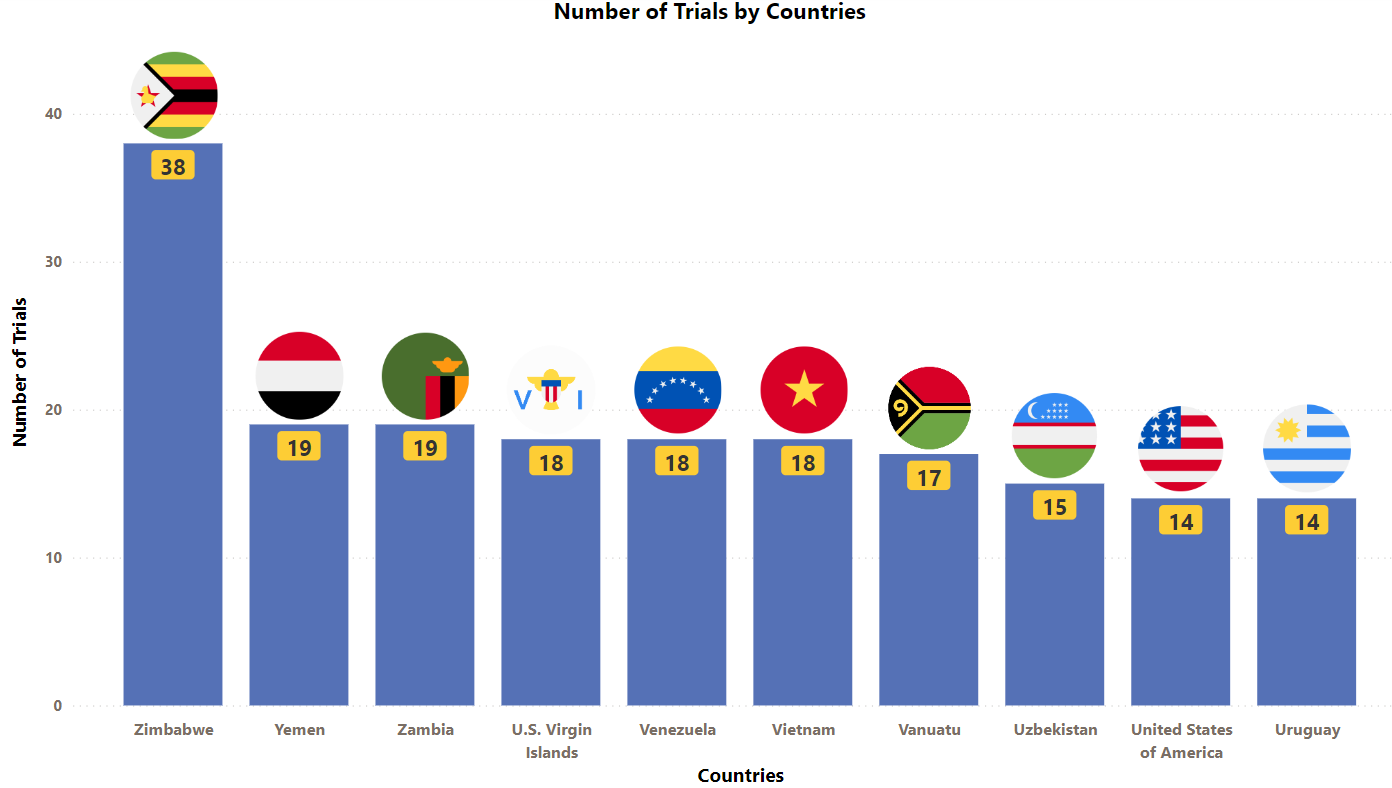 Besides the data shown in the chart, 19 countries have double-digit trial numbers, and 40 countries with single-digit trial numbers. For a detailed report on it, mail us at connect@pharmashots.com
Besides the data shown in the chart, 19 countries have double-digit trial numbers, and 40 countries with single-digit trial numbers. For a detailed report on it, mail us at connect@pharmashots.com
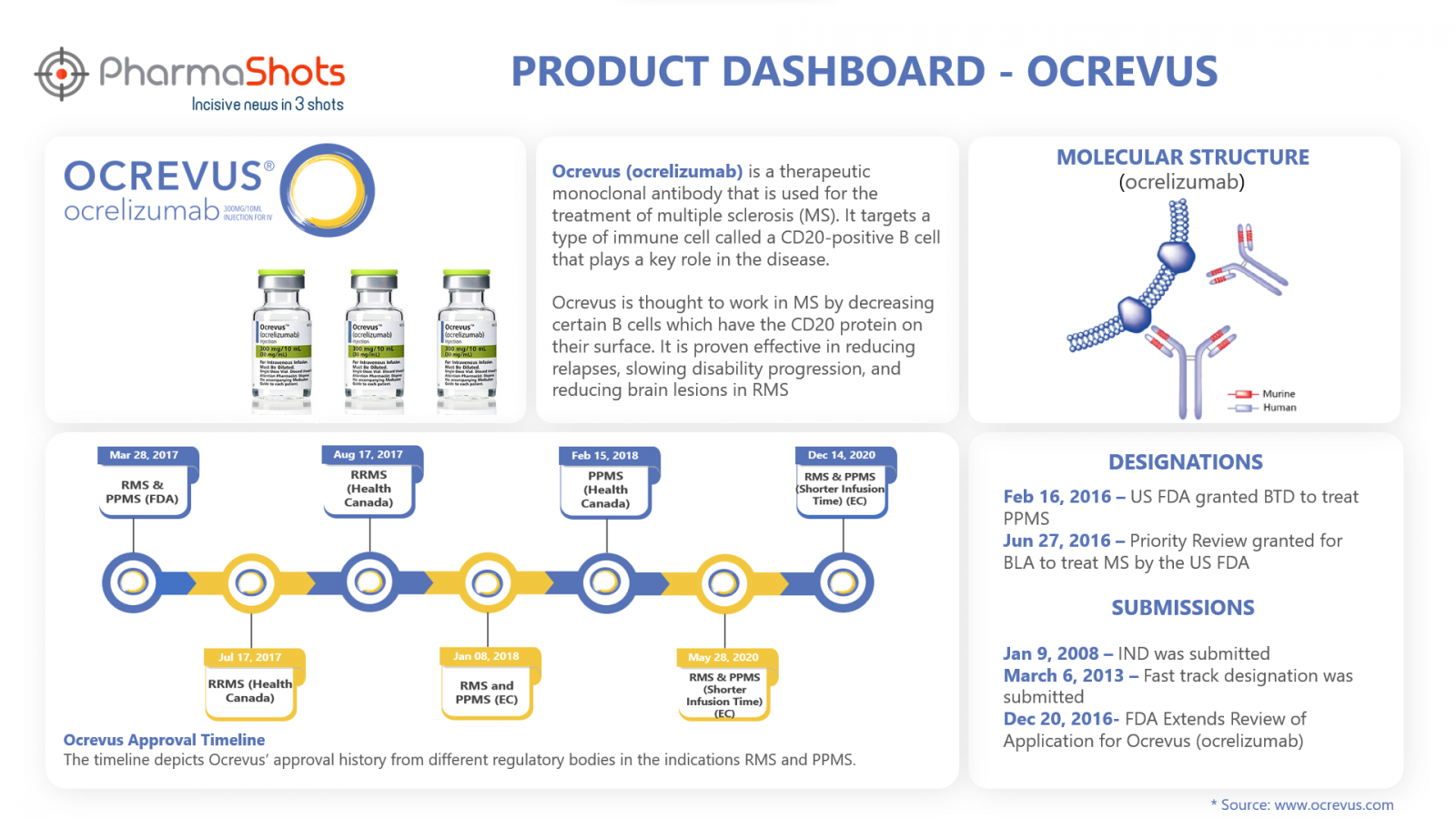
Product Dashboard
PharmaShots brings an illustrative infographic showcasing essential metrics and relevant data on Ocrevus
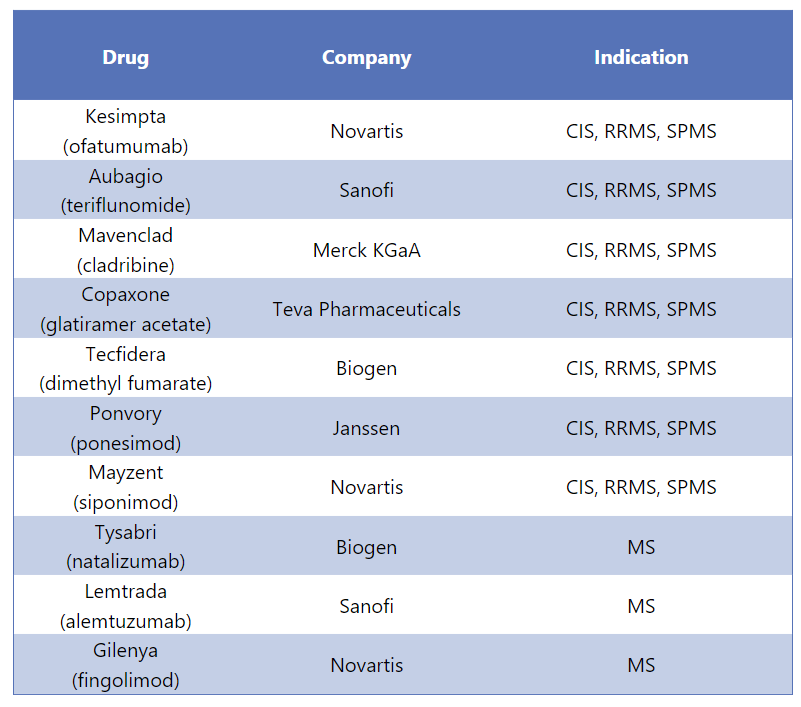
Ocrevus Alternative Drugs5
Ocrevus has various substitutes in the market that are used for different forms of MS. Some of the alternative medications for Ocrevus are mentioned below:
SWOT Analysis6
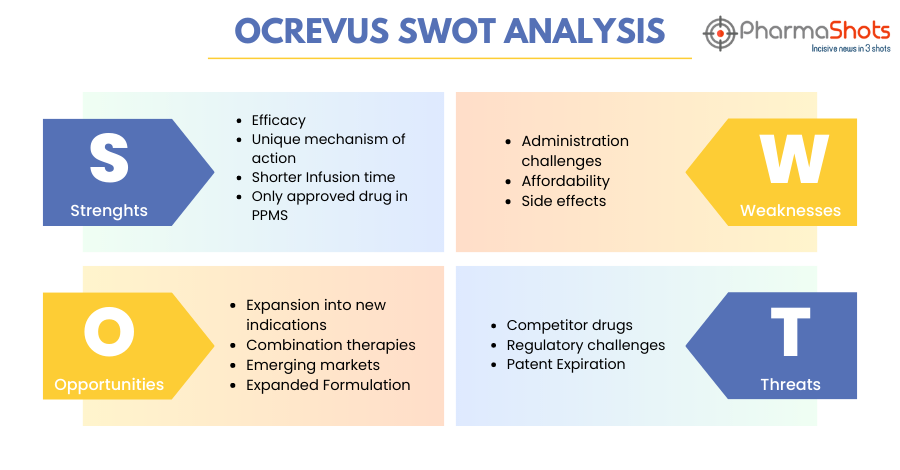
Strengths:
- Efficacy: Ocrevus has demonstrated high efficacy in the treatment of MS, particularly in reducing relapse rates and disease progression. It has been shown to be effective in both relapsing and primary progressive forms of MS. For more information on the efficacy results visit www.ocrevus.com
- Unique mechanism of action: Ocrevus targets specific immune cells involved in the progression of MS, known as CD20+ve B cells. Following cell surface binding to B lymphocytes, ocrelizumab results in ADCC and complement-mediated lysis. This targeted approach makes it different from other existing MS treatments and may offer advantages in terms of efficacy and safety.
- Shorter Infusion Time: Ocrevus is an IV formulation administered every six months and has shorter infusions for subsequent doses. Three treatments in the first year which include the first 600-mg dose administered as two 300-mg IV infusions over approximately 2.5 hours, separated by 2 weeks followed by subsequent doses administered as a single 600-mg IV infusion every 6 months
- Only approved drug in PPMS: Ocrevus is the only approved drug for primary progressive multiple sclerosis. Ocrelizumab was effective in delaying clinical progression in patients with PPMS, significantly reducing the proportion of patients with 12-week CDP relative to placebo in a clinical trial.
Weaknesses:
- Administration challenges: While the dosing schedule of Ocrevus is convenient, the intravenous administration requires visits to healthcare facilities and 2-4 hours of waiting time, which can be inconvenient for some patients
- Affordability: The high cost of Ocrevus may be unaffordable for some patients thus limiting its access.
- Side effects: Although Ocrevus has a generally favorable safety profile, there are potential side effects, including infusion-related reactions, which can be serious and may require hospitalization, increased risk of infections, and other long-term effects.
Opportunities:
- Expansion into new indications: Ocrevus can expand its market by exploring its efficacy in other autoimmune diseases or expanding its use in different subtypes of MS.
- Combination therapies: Ocrevus can be used in combination with other MS treatments, which can provide new opportunities or treatments for managing MS and its forms
- Emerging markets: Ocrevus is currently approved in over 98 countries across North America, South America, the Middle East, Europe, as well as in Australia. Its growing demand can help increase its market presence at a broader level worldwide.
- Expanded Formulation: Ocrevus is administered by intravenous injection. Its expansion in different formulations can provide ease for the patients in the administration of the drug
Threats:
- Competitor Drugs: There are several existing and emerging treatments for MS, creating a competitive market. Ocrevus faces competition from other disease-modifying therapies, some of which may offer alternative benefits to patients.
- Regulatory Challenges: Ocrevus may face regulatory challenges in some regions, which could impact its availability or market access. Obtaining regulatory approvals for new treatments and expanding indications can be a complex process
- Patent Expiration: Ocrevus has near-term patent expiry. Its US patent will expire in 2029 in the US, Germany, France, Spain, the UK, and Italy in 2028. This will create entry for generic and biosimilar competitors to enter the market, potentially eroding its market share and revenue.
Patient Stories7
Patients' stories have been instrumental in sharing their perspectives on individual experiences in facing health challenges. These are a few key resources to understand healthcare services and policies. Some of the patients’ stories for Ocrevus are mentioned below:
- ALEX’s story: Alex was diagnosed with MS when he was in his early 30s and in good health. When he went in for his follow-up appointment with his doctor, he didn’t waste any time. He immediately said, “You have MS.” Alex was in a state of shock, but his doctor just kept moving forward discussing treatments and plans. Alex says “At this point, I knew MS was serious, but I had no clue what the disease was. For me personally, infusions have become something I look forward to. It is a time where I can relax, catch up on work, or watch streaming videos.”
- GARY’s Story: Gary’s MS journey included a time when he was debilitated. Gary says “One thing I’d say is: it’s important to be happy. I know that's odd advice but hear me out—this is your way of being proactive about your MS treatment”
- JOZI’s story: Jozi says that the day she was diagnosed with MS is the one she remembers very clearly. She felt a lot of different things and was confused and really, really scared. During her infusion, she says “One thing I wish I had known before going to my first infusion is that it’s not so bad. I remember being scared, nervous, anxious, excited, and generally overwhelmed. In reality (and this is my personal experience, of course) it wasn’t bad”
*To read more about Ocrevus patient stories, visit www.ocrevus.com
KOL* Reviews8
KOL reviews offer valuable perspectives on different products and services. These reviews prove beneficial for consumers who engage in product research and prefer to read multiple reviews before making a purchase. Here are a few KOL reviews regarding Ocrevus.
- In Aug 2017, when Health Canada approved Ocrevus for the treatment of RRMS, Dr. Daniel Selchen, Neurologist and Head of the Division of Neurology at St. Michael's Hospital in Toronto said, "Ocrevus is a major addition to the treatment options available for MS. The RRMS Ocrevus clinical trial data show a significant reduction in relapses and disease progression, as well as a good safety profile, for appropriate patients, Ocrevus will be of great value in reducing the burden of MS"
- When Health Canada approved Ocrevus in August 2017 as a treatment for RRMS, Dr. Karen Lee, Vice-President of research at the Multiple Sclerosis Society of Canada said, “The approval of Ocrevus is good news for people living with MS, as they now have one more treatment option to help manage this unpredictable disease"
- In Oct 2022, when Genentech announced new data disease progression and healthcare costs in patients with early-stage RRMS, RMS, and primary PPMS, Stephen Hauser, Chair of the Scientific Steering Committee of the OPERA studies and Director of the Weill Institute for Neurosciences at the University of California, San Francisco said “Nine-year data presented at ECTRIMS in RMS and PPMS continue to show significant efficacy against disease activity and progression with a consistent long-term safety profile, which is very encouraging for patients living with this disease and their physicians, Ocrevus has significantly changed the treatment paradigm for more than 250,000 people with MS since its approval more than five years ago"
- In 2017, after its approval, Ocrevus experienced a boost in its sales. Roche CEO Severin Schwan said, "Particularly pleasing is the very successful launch of Ocrevus for the treatment of two forms of multiple sclerosis, based on our half-year performance, we raised the outlook for the full-year to mid-single-digit sales growth.”
- In Jul 2023 when Genentech announced positive P-III Results for Ocrevus Twice a Year, 10-Minute Subcutaneous Injection in Patients with Multiple Sclerosis, Levi Garraway, Genentech’s CMO and Head of Global Product Development said “This new subcutaneous injection will allow Ocrevus to be administered in 10 minutes twice a year, helping people living with MS to spend less time in treatment for this disease.’’
*Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL identification and mapping? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
Sec fillings
2. Ocrevus Prescribing Information
4. Ocrevus Trials (country-wise)
5. Ocrevus Alternative drugs
6. SWOT analysis
8. KOL reviews
Related Post: Top Performing Drug – Enbrel (June Edition)
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.














