
Insights+: The US FDA New Drug Approvals in January 2023
Shots:
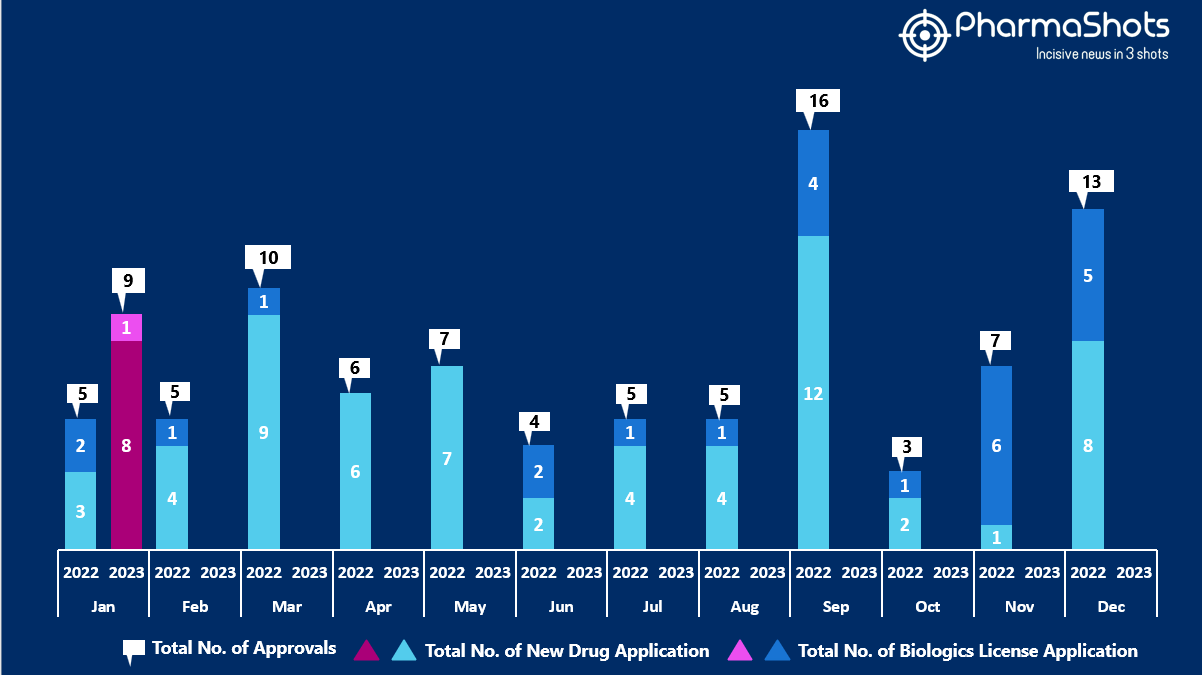
- The US FDA approved 8 NDAs and 1 BLA in January 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 9 novel products in 2023
- In January 2023, the major highlights drugs were Rykindo (risperidone) approval for schizophrenia and bipolar 1 disorder, Brenzavvy (bexagliflozin) for type 2 diabetes
- PharmaShots has compiled a list of a total of 9 new drugs approved by the US FDA in January 2023
Eisai and Biogen Receive the US FDA’s Approval of Leqembi (lecanemab-irmb) for Alzheimer's Disease
Leqembi
Active ingredient: lecanemab-irmb Approved: January 09, 2023
Company: Eisai and Biogen Disease: Alzheimer Disease
- The US FDA has approved Leqembi (100mg/mL, IV, q2w) for AD under the accelerated approval pathway. The product is expected to be available during or before Jan 23, 2023
- The approval was based on the P-II study of Leqembi (humanized IgG1 mAb) which showed a reduction in the accumulation of Aβ plaque in the brain. The company also submitted sBLA to the US FDA for approval under the traditional pathway, based on the P-III trial (Clarity AD) presented at CTAD conference & published in the NEJM
- The company launched the patient support program, providing access to patients for Leqembi treatment incl. insurance coverage & co-pay programs. At the same time, PAP offers Leqembi at no cost for eligible uninsured & underinsured patients, incl. Medicare beneficiaries
Airsupra
Active ingredient: albuterol and budesonide Approved: January 12, 2023
Company: AstraZeneca and Avillion Disease: Asthma
- The US FDA has approved Airsupra (albuterol/budesonide) for as-needed treatment or prevention of bronchoconstriction & to reduce the risk of exacerbations in patients with asthma aged ≥18yrs.
- The approval was based on the P-III trial (MANDALA) & (DENALI) evaluating PT027 vs albuterol in 3132 & 1001 adults and children aged 4-11yrs. Both trials met their 1EPs i.e., reductions in risk of sev. exacerbations over albuterol, when used as a rescue medicine in response to symptoms in (MANDALA) trial, added ICS alone or in combination with asthma maintenance therapies
- In the (DENALI) trial, lung function improved as measured by FEV1 over albuterol & budesonide. The safety & tolerability in both trials were consistent with the known profiles
Rykindo
Active ingredient: risperidone Approved: January 16, 2023
Company: Luye Pharma Disease: Schizophrenia and Bipolar 1 Disorder
- Rykindo has received approval from the US FDA for extended-release injectable suspension for the treatment of schizophrenia & as monotx. or as adjunctive therapy to lithium or valproate for bipolar I disorder in adults
- Rykindo is a long-acting risperidone inj. & marks the first innovative therapy to be marketed in the US. The therapy was administered via IV, q2w uses long-acting, extended-release microsphere technology to deliver the active component, risperidone which was approved for marketing in China for schizophrenia
- Rykindo’s development is moving forward in the EU & the company is planning to launch the therapy in more countries and regions globally
BeiGene’s Brukinsa (zanubrutinib) Receives the US FDA’s Approval for Chronic Lymphocytic Leukemia
Brukinsa
Active ingredient: zanubrutinib Approved: January 20, 2023
Company: BeiGene Disease: Chronic Lymphocytic Leukemia
- The approval was based on the results from the P-III studies (ALPINE) in 652 patients & (SEQUOIA) trial in 740 patients evaluating Brukinsa. The results showed superior efficacy & favorable safety profile
- In the (SEQUOIA) trial, the results showed a PFS benefit over bendamustine + rituximab with a median follow-up of 26.2mos. In the (ALPINE) trial, superior ORR over ibrutinib, ORR (80.4% vs 72.9%) while the overall safety profile in both trials was consistent with prior studies
- In the pre-defined final PFS analysis of the (ALPINE) study, superior PFS with a median follow-up of 29.6mos., favorable cardiac safety profile with lower rates of AF/flutter (5.2% vs 13.3%) & zero deaths due to cardiac disorders (0% vs 1.9%)
Brenzavvy
Active ingredient: bexagliflozin Approved: January 24, 2023
Company: TheracosBio Disease: Type 2 Diabetes
- The US FDA has approved Brenzavvy, an oral sodium-glucose cotransporter 2 inhibitor that is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2D
- The approval was based on the results from a clinical program in 23 clinical trials evaluating the safety & efficacy of Brenzavvy in 5000+ adult patients with T2D mellitus. The P-III studies showed a reduction in hemoglobin A1c & fasting blood sugar after 24wks. as monotx & in combination with metformin, or as an add-on to SoC treatment
- Brenzavvy will be available as 20mg oral tablets to be taken qd. The one P-II trial results showed that the therapy was well-tolerated & provide a durable & clinical improvement in glycemic control over 96wks.
Bravecto
Active ingredient: fluralaner Approved: January 27, 2023
Company: Merck Animal Health Disease: Asian Long-Horned Ticks in Dogs
- The company has expanded the indication for Bravecto Chews to include the treatment & control of Asian long-horned ticks in dogs
- Through the US FDA approval, Merck has been able to extend its Bravecto product portfolio to provide a broad-spectrum comprehensive line of parasite protection products delivering immediate & persistent killing activity in dogs
- Bravecto (112.5, 250, 500, 1000 & 1400 mg fluralaner/chew) is a chewable tablet indicated for the treatment & prevention of flea infestations, treatment & control of Amblyomma americanum infestations (for 8wks.), tick infestations, Dermacentor variabilis, Rhipicephalus sanguineus & Haemaphysalis longicornis (for 12wks.)
Keytruda
Active ingredient: pembrolizumab Approved: January 30, 2023
Company: Merck Disease: Non-Small Cell Lung Cancer
- The approval was based on the P-III trial (KEYNOTE-091) results evaluating Keytruda (200mg, IV, q3w) vs PBO in a ratio (1:1) in 1177 patients with completely resected stage IB (T2a ≥4 cm), II, or IIIA NSCLC
- The results showed an improvement in DFS who received adjuvant Pt-based CT following surgical resection, a 27% reduction in risk of disease recurrence or death, m-DFS (58.7mos. vs 34.9mos.) in patients regardless of PD-L1 expression
- In an exploratory subgroup analysis, the DFS HR was 1.25 among 167 patients who did not receive adjuvant CT while the AEs reported in the trial (KEYNOTE-091) were similar to that observed in other trials of pembrolizumab
Jaypirca
Active ingredient: pirtobrutinib Approved: January 30, 2023
Company: Eli Lilly Disease: Mantle Cell Lymphoma
- The US FDA has approved Jaypirca (100/50mg) for adult patients with r/r MCL after two lines of systemic therapy. The approval was based on the P-I/II trial (BRUIN) includes a P-I dose-escalation phase, a P-Ib combination arm, and a P-II dose-expansion phase evaluating Jaypirca in 120 patients
- The results showed that patients treated with Jaypirca (200mg) achieved an ORR (50%), CR (13%), PR (38%), and median time to response (1.8mos.) and m-DoR was 8.3mos., 6mos. DoR rate (65.3%) while 83% discontinued the last BTK inhibitor due to refractory or progressive disease. In the pooled safety analysis, adverse reactions were reported in ≥20%
- Dose reductions & treatment interruptions due to AEs in 4.7% & 32%, respectively, and permanent discontinuation of the study drug (9%)
Orserdu
Active ingredient: elacestrant Approved: January 31, 2023
Company: Context Therapeutics Disease: Mutated Breast Cancer
- The US FDA has approved Orserdu for postmenopausal women or adult men with ER+, HER2-, ESR1-mutated advanced or metastatic breast cancer. The approval was based on the P-III trial (EMERALD) results evaluating elacestrant vs SoC endocrine monotx. in 478 patients
- The trial met both 1EPs in all patients & whose tumors harbor ESR1 mutations (45% reduction in risk of progression or death) along with a significant PFS, m-PFS (8.6 vs 1.9mos.) whose tumors harbored ESR1 mutations & had been treated with a CDK4/6i for 12mos.
- ONA-XR (onapristone extended release), an oral progesterone receptor antagonist is being tested in the ongoing P-Ib/II trial (ELONA) to improve the activity of Orserdu in ESR1-mutated & wild-type metastatic breast cancer
Related Post: Insights+: The US FDA New Drug Approvals in December 2022
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














