
Insights+: The US FDA New Drug Approvals in December 2022
Shots:
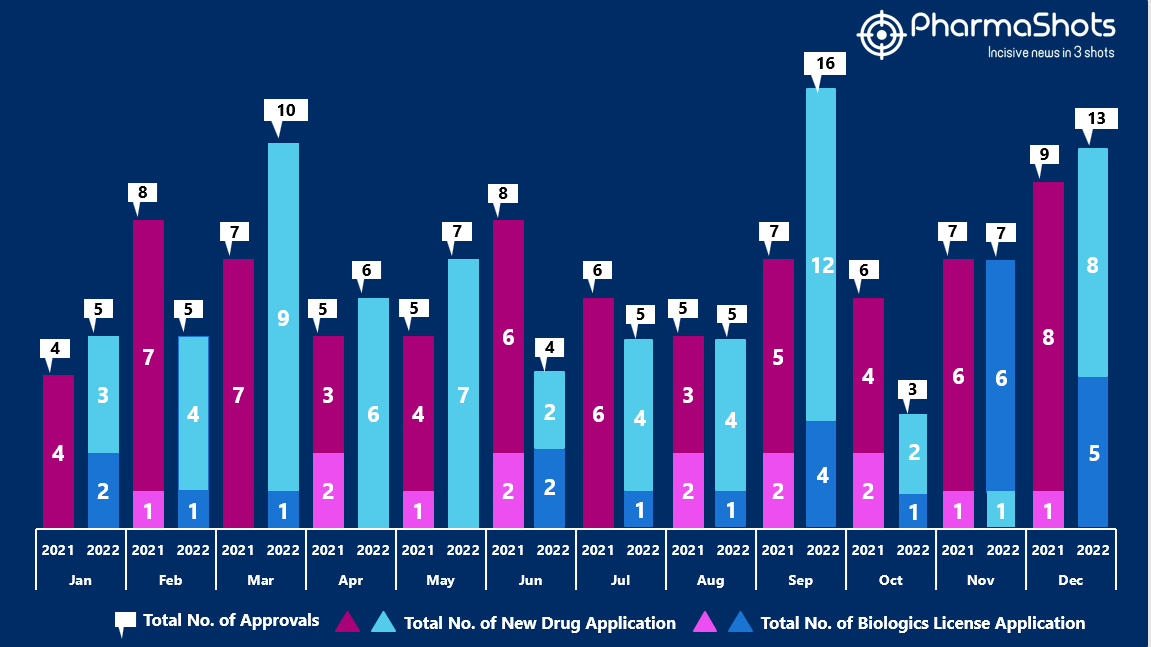
- The US FDA approved 8 NDAs and 5 BLA in December 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 86 novel products in 2022
- In December 2022, the major highlights drugs were Krazati (adagrasib) approval for non-small cell lung cancer with a KRASG12C mutation, Sunlenca (lenacapavir) for multi-drug resistant HIV
- PharmaShots has compiled a list of a total of 13 new drugs approved by the US FDA in December 2022
Brexafemme
Active ingredient: ibrexafungerp Approved: December 02, 2022
Company: SCYNEXIS Disease: Recurrent Vulvovaginal Candidiasis
- The US FDA has approved Brexafemme for the reduction in the incidence of recurrent vulvovaginal candidiasis
- The approval was based on the P-III (CANDLE) study evaluating the safety & efficacy of ibrexafungerp vs PBO showed that 65.4% vs 53.1% of patients treated with ibrexafungerp achieved clinical success with no recurrence either culture-proven, presumed, or suspected @24wk. The advantage was sustained over 3mos. follow-up period and remained statistically significant, was generally safe and well-tolerated
- The results were generally consistent with the prior Brexafemme label. Brexafemme is a novel oral antifungal that has been approved in the US in June 2021 for vulvovaginal candidiasis
Rezlidhia
Active ingredient: olutasidenib Approved: December 02, 2022
Company: Rigel Disease: Acute Myeloid Leukemia
- The US FDA has approved Rezlidhia for patients with r/r AML with a susceptible IDH1 mutation
- The approval was based on the P-II study evaluating Rezlidhia (150mg, BID) as monothx. in 153 patients which showed that patients achieved a 35% CR+CRh rate with an m-DoR of 25.9mos., the median time to CR or CRh was 1.9mos. Among patients who achieved the 1EPs of CR+CRh, 92% were CR with an m-DoR of 28.1mos.
- The therapy was well tolerated, 16% reported differentiation syndrome & which was manageable with dose interruption & corticosteroids. Under Aug 2022 collaboration with Forma, Rigel will be responsible to launch & commercialize Rezlidhia in the US & is planning to work with partners for developing & commercializing the product outside the US
Krazati
Active ingredient: adagrasib Approved: December 07, 2022
Company: Mirati Therapeutics Disease: Non-Small Cell Lung Cancer
- The approval was based on the P-II study (KRYSTAL-1) evaluating Krazati (600mg, BID) in 116 patients with KRASG12C-mutated advanced NSCLC which showed a positive benefit-risk profile, ORR (43%) with 80% of patients achieved disease control & m-DoR was 8.5mos.
- In a pooled efficacy analysis incl. P-I/Ib NSCLC & P-II NSCLC cohorts from the (KRYSTAL-1) study, the results showed an ORR (44%), DCR (81%) based on BICR, m-DoR of 12.5mos. and m-OS of 14.1mos., AEs leading to permanent discontinuation (13%)
- The company launches Mirati & Me, a comprehensive program to provide access to patients, caregivers & oncology community to use the treatment & also offers financial, educational & emotional support services to eligible patients
Idacio
Active ingredient: adalimumab Approved: December 14, 2022
Company: Fresenius Kabi Disease: Chronic Autoimmune Diseases
- The US FDA has approved citrate-free adalimumab biosimilar, Idacio for chronic autoimmune diseases. The product is expected to be available in the US in July 2023 as a self-administered prefilled syringe and pre-filled pen (autoinjector)
- The approval was based on a review of a comprehensive data package & totality of evidence that demonstrated a similar analytical profile, PK, efficacy, safety, and immunogenicity over the adalimumab product
- Idacio is a tumor necrosis factor (TNF) blocker and a biosimilar to Humira. The product has been approved and commercialized in ~37 countries globally & has been developed by Fresenius Kabi SwissBioSim by using advanced analytical methods for multiple chronic conditions
5. Thea Pharma’s Iyuzeh Receives the US FDA’s Approval for Open-Angle Glaucoma or Ocular Hypertension
Iyuzeh
Active ingredient: N/A Approved: December 14, 2022
Company: Thea Pharma Disease: Hypertension
- The US FDA has approved the NDA of Iyuzeh (latanoprost ophthalmic solution) 0.005% for the reduction of elevated IOP in patients with OAG or ocular hypertension (OHT). Iyuzeh is expected to be available for US eyecare practitioners in H2’23
- The clinical trials results showed that Iyuzeh lowered IOP by 3-8 mmHg vs 4-8mmHg with latanoprost ophthalmic solution (Xalatan; Viatris) 0.005%, which contains benzalkonium chloride in patients with open-angle glaucoma or ocular hypertension with a mean baseline IOP of 19-24mmHg
- Iyuzeh is the first preservative-free formulation of latanoprost in the US that reduces IOP ~3-4 hrs. after administration with a maximum effect observed after 8-12hrs.
Adstiladrin
Active ingredient: nadofaragene firadenovec-vncg Approved: December 16, 2022
Company: Ferring Disease: Bladder Cancer
- The US FDA has approved Adstiladrin for the treatment of adult patients with high-risk, BCG-unresponsive NMIBC with carcinoma in situ with/out papillary tumors. The therapy is expected to be available in the US in H2’23
- The approval was based on the P-III study results evaluating the efficacy and safety of Adstiladrin (q3mos.) in 157 patients. The trial met its 1EPs which showed that 51% of CIS ± Ta/T1 patients achieved a CR @3mos. & were remain free of high-grade recurrence (46%) @12mos.
- Adstiladrin is a novel adenovirus vector-based gene therapy & has been studied in a clinical trial program in the same indication who had prior been treated with adequate BCG
Tymlos
Active ingredient: abaloparatide Approved: December 21, 2022
Company: Radius Health Disease: Osteoporosis
- The US FDA has approved Tymlos for the treatment of men with osteoporosis who are at high risk of fracture, who have failed or are intolerant to other available osteoporosis therapy
- The approval was based on the P-III study (ATOM) evaluating abaloparatide (80ug) vs PBO in a ratio (2:1) in 228 patients with osteoporosis. The 1EPs of the study was the percent change from baseline in bone mineral density at the lumbar spine (8.5% vs 1.2%) @12mos., treatment difference was 7.3% while the 2EPs incl. the percent change from baseline in BMD at the total hip & femoral neck @12mos.
- Tymlos has been approved in the US in April 2017, based on the P-III study (ACTIVE) for postmenopausal women with osteoporosis
8. Gilead’s Sunlenca (lenacapavir) Receives the US FDA’s Approval for Multi-Drug Resistant HIV
Sunlenca
Active ingredient: lenacapavir Approved: December 22, 2022
Company: Gilead Disease: HIV
- The US FDA has approved Sunlenca (HIV capsid inhibitor) + other antiretroviral(s) for HIV-1 inf. in 36 heavily treatment-experienced adults with MDR HIV-1 inf.
- The approval was based on the P-II/III trial (CAPELLA) results evaluating lenacapavir (q6mos., SC) + optimized background regimen in a ratio (2:1) at research centers in North America, EU & Asia where 83% achieved an undetectable viral load (<50 copies/mL) @52wk. with a mean increase in CD4 count of 82 cells/µL. The results were presented at CROI 2022
- The therapy received BTD from the US FDA for HIV-1 inf. The EMA will be valid in 27 member states of the EU, Norway, Iceland & Liechtenstein while additional regulatory filings & regulatory authorities’ decisions are expected in 2023
Lunsumio
Active ingredient: mosunetuzumab-axgb Approved: December 23, 2022
Company: Genentech Disease: Follicular Lymphoma
- The US FDA has approved Lunsumio (CD20xCD3 T-cell engaging bispecific Ab) for r/r FL after ≥2 lines of systemic therapy. The product can be administered as an IV inf. for a fixed duration & expected to be available in the US in the coming weeks
- The approval was based on the P-II (GO29781) dose-escalation & expansion study evaluating Lunsumio in 836 patients with heavily pretreated FL who were at high risk of disease progression or whose disease was refractory to prior therapies
- The results showed high & durable response rates, ORR (80%) with a majority of patients-maintained responses for 18mos, m-DoR was (22.8mos.), CR (60%). The effects of lunsumio on earlier line therapies for non-lymphoma Hodgkin's patients are being studied in P-III studies as an SC formulation
Olpruva
Active ingredient: sodium phenylbutyrate Approved: December 27, 2022
Company: Acer Therapeutics Disease: Urea Cycle Disorders
- The US FDA has approved Olpruva (sodium phenylbutyrate) as an oral suspension for UCDs. The therapy was developed by Acer & its collaboration partner Relief Therapeutics
- The approval was based on the 2 bioequivalence trials results evaluating Olpruva vs Buphenyl powder which showed similar relative bioavailability for phenylbutyrate & phenylacetate vs Buphenyl (sodium phenylbutyrate). The results were presented at SIMD 2022 & the GMDI Conference 2022
- Olpruva is supplied in dosage strengths of 2/3/4/5/6/6.67g of sodium phenylbutyrate. Acer intends to offer patient support services to facilitate access to therapy while navigator is designed to assist UCD patients with support, access, education, and adherence
Xenoview
Active ingredient: xenon Xe 129 hyperpolarized Approved: December 28, 2022
Company: Polarean Disease: Lung Disease
- The US FDA has approved Xenoview, a hyperpolarized contrast agent indicated for use with MRI for lung ventilation in adults & pediatric patients aged ≥12yrs. The product is administered by oral inhalation as a single 10-15 second breath hold MRI procedure
- The approval was based on the P-III trial (Study 1 & 2) evaluating Xenoview MRI vs xenon Xe 133 scintigraphy in 80 patients. (Study 1) patients evaluated for lung resection surgery & in (Study 2) for lung transplant surgery
- Both the trial met their 1EPs i.e., the (Study 1 &2) trial results showed observed estimation of 1.4% & 1.6% for the mean within-patient difference was within a predetermined equivalence interval in the postoperative percentage of remaining & overall lung ventilation b/w Xenoview & xenon Xe 133 imaging
Briumvi
Active ingredient: ublituximab-xiiy Approved: December 29, 2022
Company: TG Therapeutics Disease: Multiple Sclerosis
- The US FDA has approved Briumvi for adult patients with RMS incl. clinically isolated syndrome, relapsing-remitting disease & active secondary progressive disease. The product is expected to be available in the US from Q1’23
- The approval was based on the P-III trials (ULTIMATE I & II) results evaluating Briumvi (IV Day 1 (150mg), Day 15 (450mg), followed by 450mg, q24w) vs teriflunomide (14mg, qd) in 1094 patients for 96wks. across 10 countries
- The results showed superiority over teriflunomide, the relative reduction in ARR in both trials (59% & 49%); no. of T1 Gd-enhancing/MRI (97% & 97%); T2 hyperintense lesions/MRI (92% & 90%), patients with 12wk. confirmed disability progression (5.2% vs 5.9%) in the combined trial
13. Vericel and MediWound Receive the US FDA’s Approval of NexoBrid for Severe Thermal Burns
NexoBrid
Active ingredient: anacaulase-bcdb Approved: December 30, 2022
Company: Vericel Disease: Thermal Burns
- The US FDA has approved NexoBrid for the removal of eschar in adults with deep partial-thickness and/or full-thickness thermal burns with expected availability in Q2’23 in the US
- The approval was based on the pre-clinical & clinical studies, incl. the P-III study (DETECT) of NexoBrid. The study met its 1EPs of incidence of ≥95% eschar removal over gel vehicle & 2EPs incl. shorter time to eschar removal, lower incidence of surgical eschar removal, non-inferiority in time to >95% wound closure was achieved
- NexoBrid was approved in 43 countries, incl. the EU, Japan, India & other international markets. Under the collaboration with Vericel, MediWound will receive a $7.5M milestones & Vericel holds an exclusive license to commercialize NexoBrid in North America
Related Post: Insights+: The US FDA New Drug Approvals in November 2022
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














