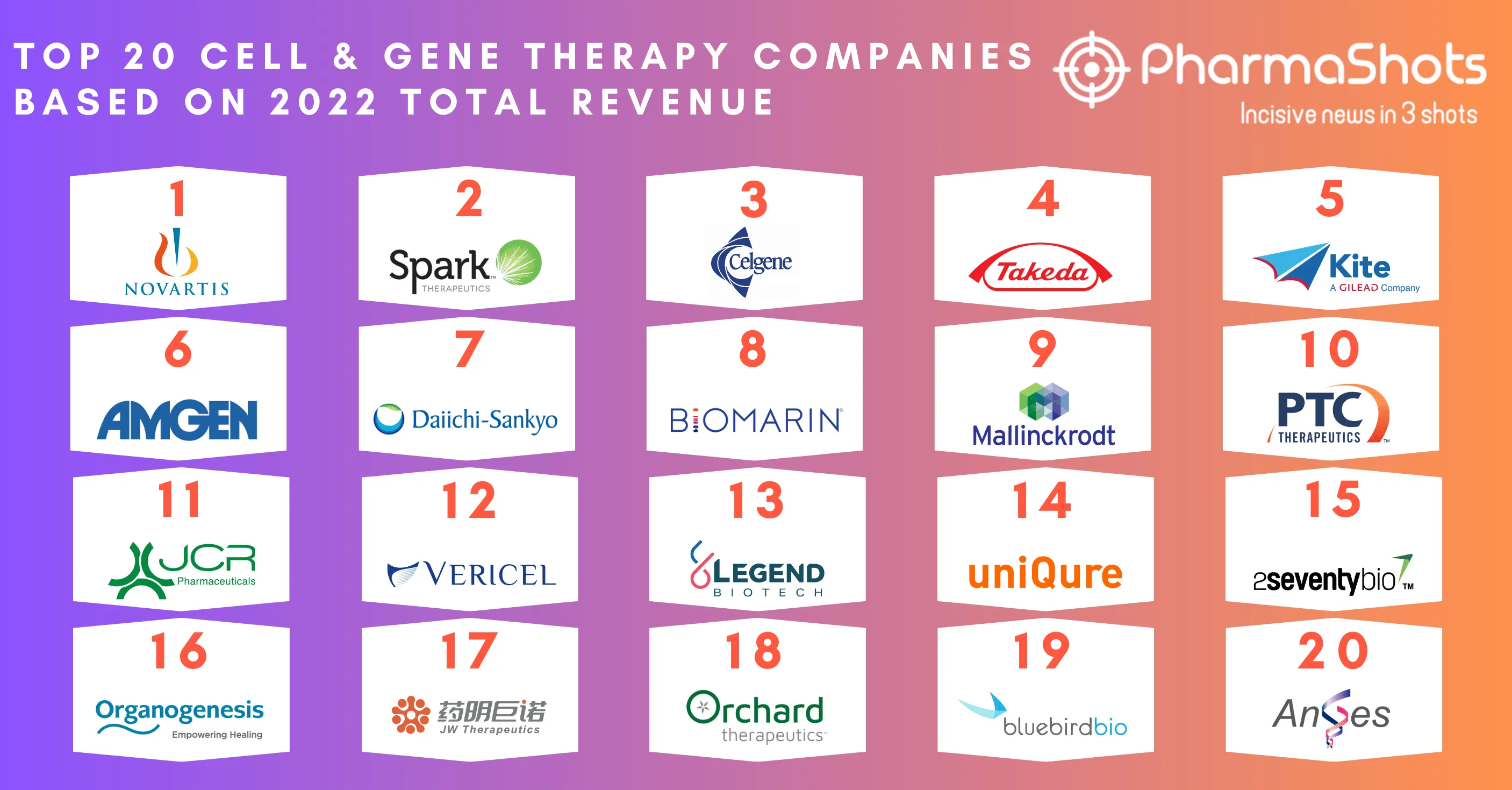
JCR Pharmaceuticals’ JR-141 Receives the US FDA’s Rare Pediatric Disease Designation for the Treatment of Mucopolysaccharidosis Type II
Shots:
- The US FDA has granted RPDD for JR-141, a recombinant fusion protein of an Ab against the human transferrin receptor and idursulfase to treat Mucopolysaccharidosis type II
- The clinical studies showed the positive effects of JR-141 on CNS symptoms. The company is currently conducting a P-III clinical trial of JR-141 in the US, Brazil, and the EU
- Under the 2021 collaboration, Takeda was responsible to commercialize JR141 outside of the US incl. Canada, EU, and other regions (Ex-Japan and other Asia-Pacific countries) & also received an exclusive license option to commercialize JR-141 in the US, following the completion of the P-III program
Ref: JCR Pharmaceuticals | Image: JCR Pharmaceuticals
Related News:- Abbott’s Navitor Device Receives the US FDA’s Approval for the Treatment of Aortic Stenosis
Click here to read the full press release
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.