Amylyx Pharmaceuticals Entered into an Exclusive Distribution Agreement with Neopharm for AMX0035
Shots:
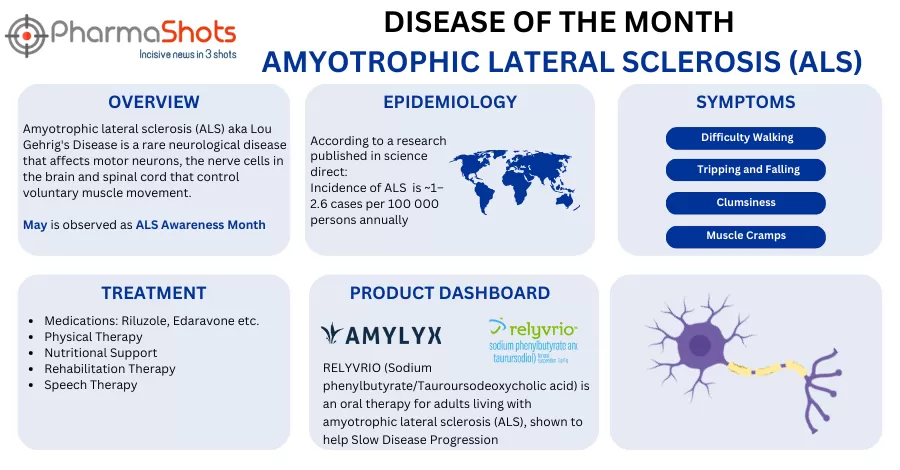
- Neopharm to get an exclusive right to commercialize AMX0035 in Israel, Gaza, West Bank & Palestinian Authority and will lead the regulatory filings and obligations for the registration and reimbursement of AMX0035 for amyotrophic lateral sclerosis
- The companies’ focus is to provide patients access to use AMX0035 & offers benefits to ALS patients. Additionally, the therapy continues to advance for the potential treatment of other neurodegenerative diseases
- AMX0035 is an oral, fixed-dose therapy that was approved to treat ALS in adults as Relyvrio (sodium phenylbutyrate & taurursodiol) in the US & as Albrioza (sodium phenylbutyrate and ursodoxicoltaurine) in Canada
Ref: Businesswire | Image: Amylyx
Related News:- Amylyx Publishes Results of AMX0035 in P-II (CENTAUR) Trial for the Treatment of Amyotrophic Lateral Sclerosis in Muscle & Nerve
Click here to read the full press release

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.