
Top Performing Drug of 2021 – Opdivo (December Edition)
Active Ingredient: Nivolumab
Strength: 40 mg/4 mL, 100 mg/10 mL, & 240 mg/24 mL solution in a single-dose vial
Dosage Form: Injection
Mechanism of Action: Programmed cell death-1 receptor antagonists (PD-1)
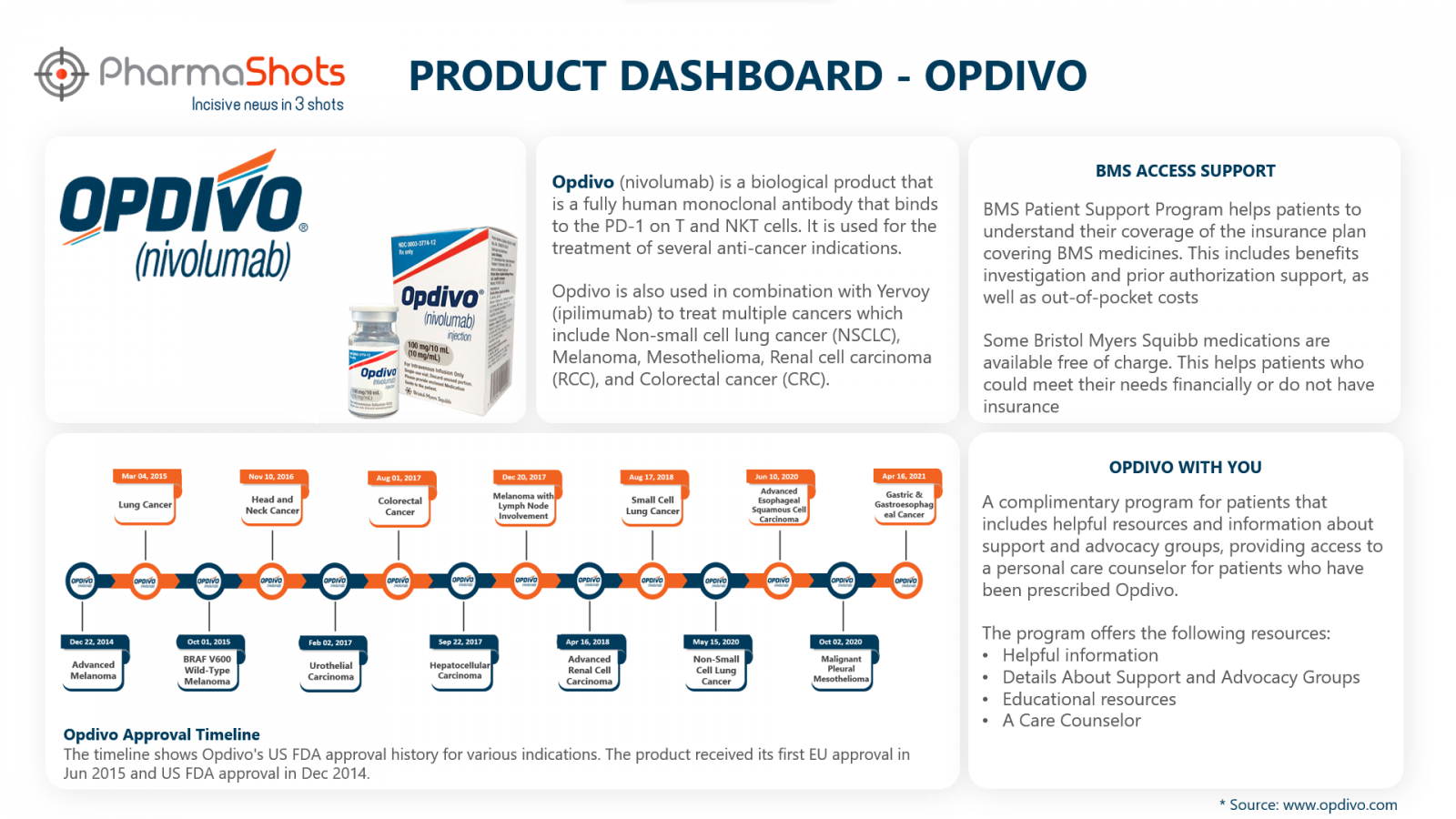
First Approval: US (22 Dec 2014), EU (19 Jun 2015)
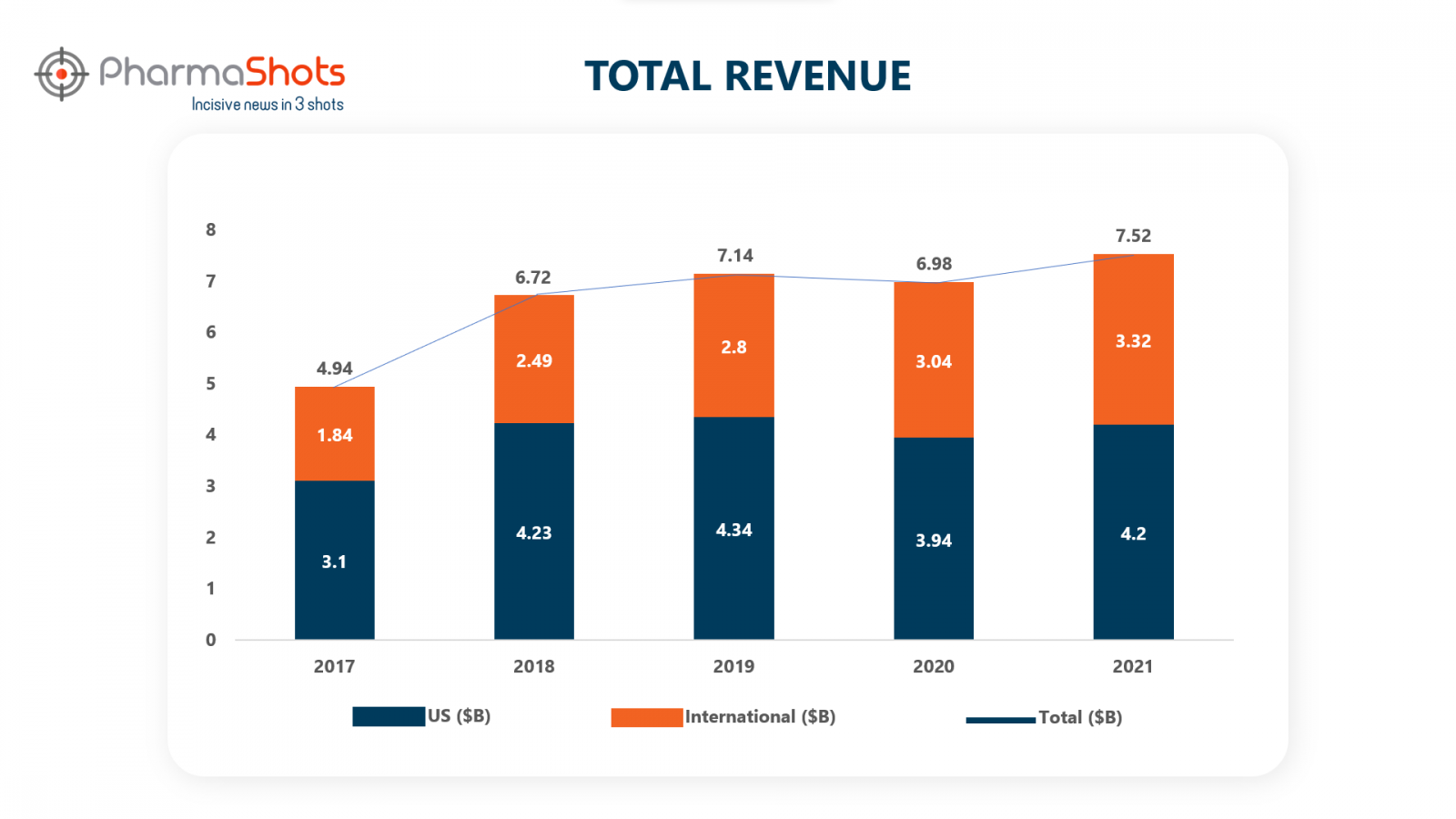
Revenue Analysis of Opdivo
BMS’ Opdivo gives tough competition to other drugs in the oncology field. Due to higher demand across multiple therapies, its revenue has increased by 8% in 2021 vs. 2020. Below is the revenue analysis done by PharmaShots showing the increasing sales of Opdivo in the past five years.
Approved Indications of Opdivo
Opdivo (nivolumab) is a fully human monoclonal antibody that binds to the PD-1 on T and NKT cells. It is used to treat patients with:
-
BRAF V600 wild-type unresectable or metastatic melanoma, as a single agent
-
BRAF V600 mutation-positive unresectable or metastatic melanoma, as a single agent
-
Melanoma with lymph node involvement or metastatic disease who have undergone complete resection, in the adjuvant setting
-
Metastatic non-small cell lung cancer and progression on or after platinum-based chemotherapy
-
Advanced renal cell carcinoma who has received prior anti-angiogenic therapy
-
Patients with intermediate or poor risk, previously untreated advanced renal cell carcinoma, in combination with ipilimumab
-
Classical Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem cell transplantation (HSCT) and brentuximab vedotin, or 3 or more lines of systemic therapy that includes autologous HSCT
-
Recurrent or metastatic squamous cell carcinoma of the head and neck with disease progression on or after a platinum-based therapy
-
Locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy and have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy
-
Adult and pediatric patients with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan
-
Hepatocellular carcinoma who has been previously treated with sorafenib
Clinical Trials Analysis
Opdivo has a total of 1638 trials, including 977 industry trials of which 906 are interventional, 66 observational & 5 are expanded access trials. The analysis of industry trials through a representation is shown below (Trials are taken as of 15 Dec 2022)
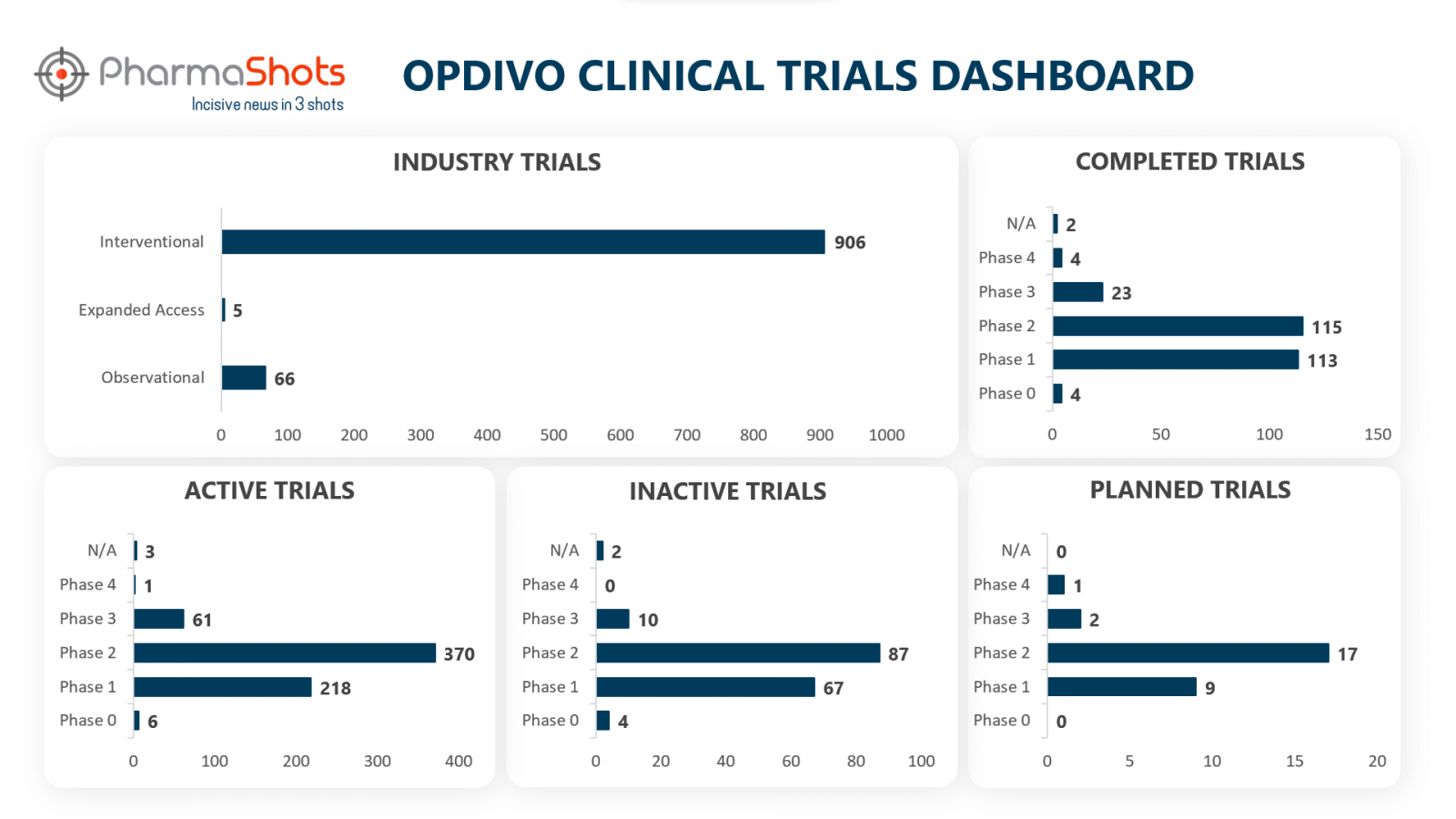
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
*Planned trials include Not, yet recruiting
Patient Assistance & Co-pay Programs for Opdivo
BMS Access Support
The program is committed to helping patients to gain access to their prescribed BMS medications.
The program provides resources to help patients understand their insurance coverage and find information on sources of financial support, including co-pay assistance for eligible commercially insured patients.
BMS Expanded Patient Support Program
This expanded patient support program is to help eligible unemployed patients in the U.S. who have lost their health insurance due to the COVID-19 pandemic. The expanded program offers access to any branded BMS medicine for free. The program covers many branded products of BMS including Opdivo.
Health Well Foundation Co-pay Program
The program helps underinsured people with life-threatening, chronic, and rare diseases get the medication and treatment they need. The program assists patients with co-pays, premiums, deductibles, and out-of-pocket expenses. It covers various medications for cancer including Opdivo.
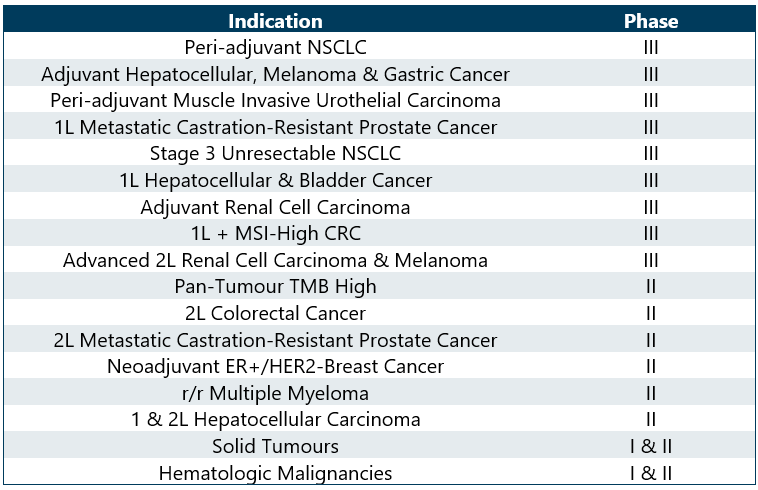
Opdivo Pipeline Analysis
Opdivo alone and in combination is being studied in multiple phases for various indications and in different lines of treatment. PharmaShots has done a detailed analysis of the BMS Opdivo’s Pipeline.
Patents
Patents provide the innovator with the right to exclude others from practicing an invention related to medicine. Opdivo has been approved in multiple markets for several anti-cancer indications. Due to these, the drug faces a big generic pressure. Opdivo's patent will expire in 2028, while the rest will lose protection by 2026.
Opdivo Competitors
Opdivo faces tough competition in the oncology space. Below mentioned is a comparative view of the competitors for Opdivo that are used for the treatment of different cancers.
|
Drug Name |
Company |
Mechanism of Action (MOA) |
|
Bavencio (avelumab) |
Pfizer |
Programmed cell death-1 ligands-1 inhibitors |
|
Yervoy (ipilimumab) |
Bristol Myers Squibb |
Cytotoxic T-lymphocyte antigen 4 inhibitors |
|
Keytruda (pembrolizumab) |
Merck & Co. |
PD-1 ligands antagonists |
|
Cabometyx (cabozantinib) |
Exelixis |
Receptor Tyrosine Kinase Inhibitors |
|
Adcetris (brentuximab vedotin) |
Seagen |
CD30-directed Antibody Interactions |
|
Nexavar (sorafenib) |
Bayer |
Protein Kinase Inhibitors |
References:
1. BMS 10-K
2. Opdivo Prescribing information
5. BMS Expanded Patient Support Program
6. Health Well Foundation Co-pay Program
7. BMS Pipeline
Related Post: Top Performing Drug of 2021 – Xarelto (November Edition)
Tags

Senior Editor at PharmaShots. She is curious and very passionate about recent updates and developments in the life sciences industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots.














