Insights+: The US FDA New Drug Approvals in November 2022
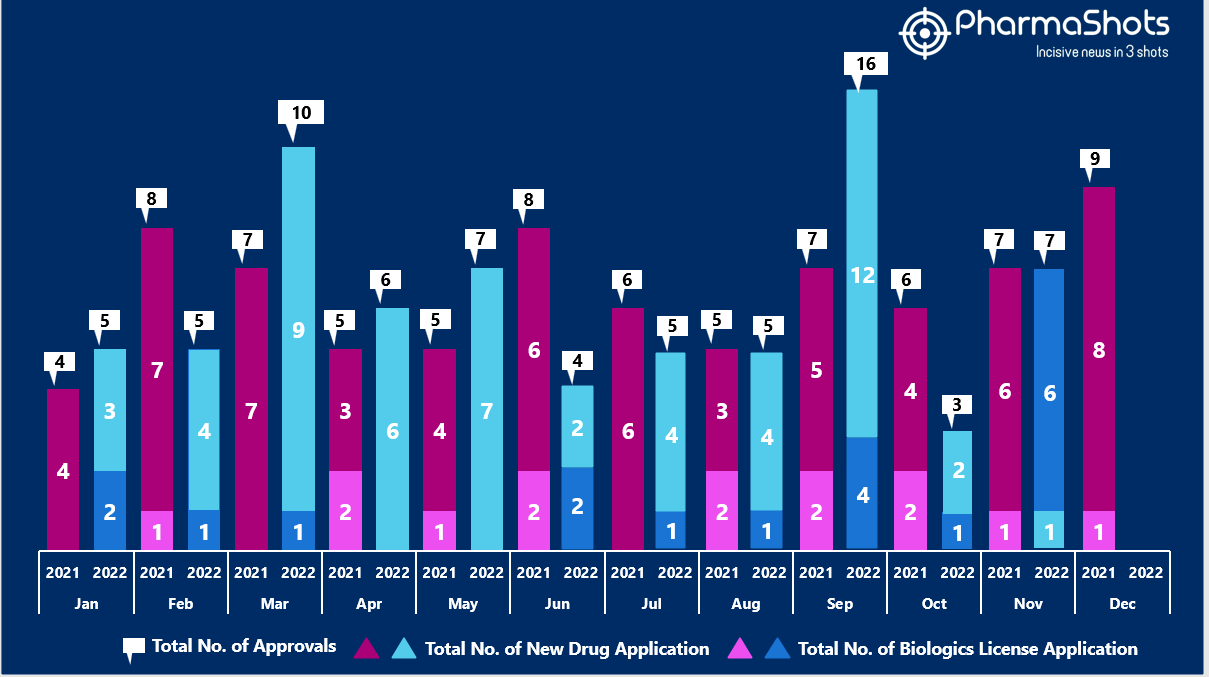
- The US FDA approved 1 NDAs and 6 BLA in November 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 73 novel products in 2022
- In November 2022, the major highlights drugs were Imfinzi (durvalumab) + Imjudo (tremelimumab) approval for metastatic non-small cell lung cancer, Elahere for platinum-resistant ovarian cancer
- PharmaShots has compiled a list of a total of 7 new drugs approved by the US FDA in November 2022
Libtayo
Active ingredient: cemiplimab Approved: November 09, 2022
Company: Regeneron Disease: Non-Small Cell Lung Cancer
- The approval was based on the P-III (EMPOWER-Lung 3) trial evaluating Libtayo (350mg, IV, q3w) + Pt-doublet CT vs CT alone in a ratio (2:1) in 466 patients with LA or metastatic NSCLC irrespective of PD-L1 expression or tumor histology with no ALK, EGFR or ROS1 aberrations
- The results showed an improvement in OS, m-OS & m-PFS (22 vs 13mos.) & (8 vs 5mos.) with 29% & 44% relative reduction in risk of death & disease progression; 12mos. probability of survival (66% vs 56% & 38% vs 16%), no new Libtayo safety signals were reported
- The therapy showed an ORR (43% vs 23%), m-DoR (16 vs 7mos.), serious AEs were reported in 25% & AEs also lead to treatment discontinuations in 5%
Adcetris
Active ingredient: brentuximab vedotin Approved: November 10, 2022
Company: Seagen Disease: Hodgkin Lymphoma
- The US FDA has approved Adcetris for high-risk cHL in combination with doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide for pediatric patients aged ≥2yrs.
- The approval was based on the P-III (NCI)-sponsored study (AHOD1331), conducted by the Children’s Oncology Group (COG) & funded by NCI evaluating Adcetris + CT (AVE-PC) vs CT (ABVE-PC) in 587 patients aged 2-21yrs. across 151 institutions. The results showed a superior EFS over ABVE-PC & 59% reduction in risk of disease progression or relapse, second cancer, or death
- Seagen & Takeda collaborated to develop Adcetris where Seagen has the US & Canadian commercialization rights, and Takeda has rights to commercialize the product globally
Imfinzi
Active ingredient: durvalumab Approved: November 14, 2022
Company: AstraZeneca Disease: Non-Small Cell Lung Cancer
- The approval was based on the P-III (POSEIDON) trial evaluating Imfinzi + Imjudo and CT vs CT alone in 1013 patients with metastatic NSCLC
- The results showed that the patients treated with the combination therapy achieved a 23% reduction in risk of death, patients were alive @2yrs. (33% vs 22%), 28% reduction in risk of disease progression or death, improvement in OS (25%), patients were alive @3yrs. (25% vs 13.6%). The safety profile was consistent with the known profiles of each medicine with no new safety signals. The results were presented at ESMO 2022 & published in the Journal of Clinical Oncology
- Based on the (POSEIDON) trial results, the regulatory application is currently under review in the EU, Japan & multiple other countries
Elahere
Active ingredient: mirvetuximab soravtansine Approved: November 14, 2022
Company: ImmunoGen Disease: Ovarian Cancer
- The approval was based on the P-III (SORAYA) trial evaluating Elahere in 106 patients with Pt-resistant ovarian cancer whose tumors expressed high levels of FRα & treated with one to three prior systemic treatment regimens & required to have received Avastin
- The results showed an ORR (31.7%) by the investigator incl. CR rate (4.8%) & PR rate (26.9%), m-DoR was 6.9mos., DCR (51.4%), 71.4% reduction in tumor size. The safety has been evaluated in a pooled analysis across 3 studies
- The therapy is also being evaluated in the (MIRASOL) trial with expected results in early 2023. The US FDA has also approved VENTANA FOLR1 (FOLR1-2.1) RxDx assay for use as a CDx device to identify patients eligible for Elahere
5. Sun Pharmaceutical’ Sezaby Receives the US FDA’s Approval for the Treatment of Neonatal Seizures
Sezaby
Active ingredient: phenobarbital Approved: November 18, 2022
Company: Sun Pharmaceutical Disease: Seizures
- The US FDA has approved Sezaby (phenobarbital sodium powder for injection) for the treatment of neonatal seizures with expected availability in the US in Q4’23
- The approval was based on the results from the P-II (NEOLEV2) study evaluating levetiracetam vs phenobarbital in 94 neonates with neonatal seizures. Patients treated with levetiracetam were seizure-free (73% vs 25%) after 24hrs.
- SEZABY is a benzyl alcohol-free and propylene glycol-free formulation of phenobarbital sodium powder for injection & marks the first product for neonatal seizures in term and preterm infants in the US. The therapy received ODD from the US FDA for the same indication
TZIELD
Active ingredient: teplizumab-mzwv Approved: November 21, 2022
Company: Provention Bio Disease: Type 1 Diabetes
- The US FDA has approved the BLA for TZIELD (anti-CD3-directed Ab) in adult and pediatric patients aged ≥8yrs. with stage 2 T1D. TZIELD inj. is provided as a sterile, preservative-free, clear & colorless solution in a 2mg/2mL (1mg/mL) single-dose vial for IV use
- Under the terms of a co-promotion agreement with Sanofi in Oct 2022, the companies collaborated to launch TZIELD in the US for the delay in the onset of clinical T1D in at-risk individuals
- The company has launched a patient support program i.e., COMPASS that provides access to patients to use TZIELD & also offers financial assistance options i.e., copay assistance for out-of-pocket costs to eligible patients
Hemgenix
Active ingredient: etranacogene dezaparvovec Approved: November 23, 2022
Company: uniQure Disease: Hemophilia B
- The US FDA has approved Hemgenix (etranacogene dezaparvovec-drlb) in adults aged ≥18yrs. with hemophilia B
- The approval was based on the P-III (HOPE-B) trial evaluating Hemgenix (2×1013gc/kg, IV) in 54 patients which showed a reduction in the rate of annual bleeds & 94% of patients discontinued factor IX prophylaxis and remained prophylaxis-free with mean factor IX activity of 39% @6mos. and 36.7% @24mos. post-infusion, 54% reduction in ABR bleeds over 6mos. on factor IX prophylactic replacement therapy
- Under the terms of the agreement, CSL gets an exclusive right globally to Hemgenix in May 2021 & responsible for the development, registration & commercialization of the therapy
Related Post: Insights+: The US FDA New Drug Approvals in October 2022





