
Insights+: EMA Marketing Authorization of New Drugs in September 2022
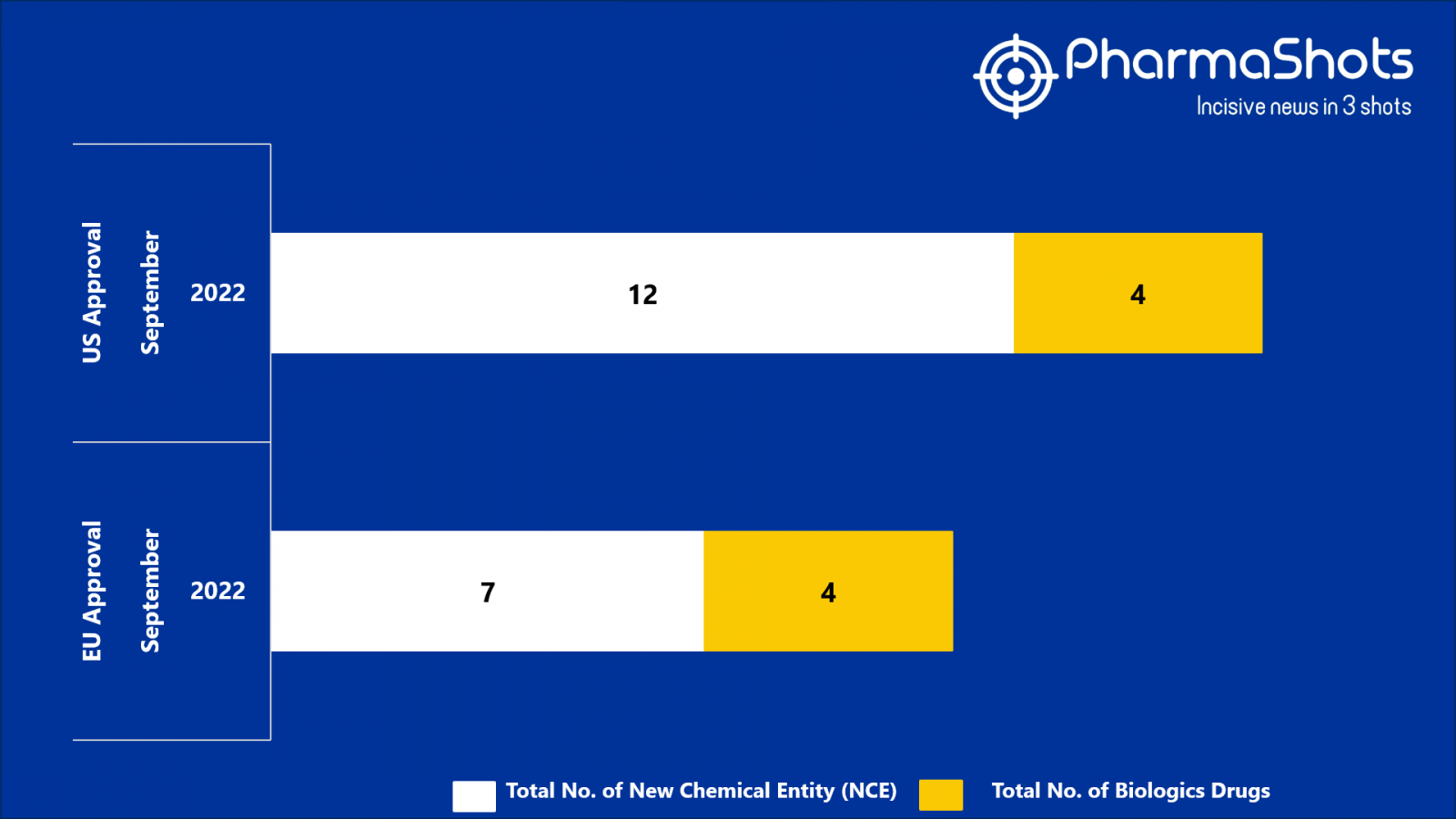
- The EMA approved 7 New Chemical Entity (NCE) and 4 Biologic Drugs in Sept 2022, leading to treatments for patients and advances in the healthcare industry
- In September 2022, the major highlights drugs were Imcivree approval for bardet-biedl syndrome, Opdualag for unresectable or metastatic melanoma, Ultomiris for generalised myasthenia gravis
- PharmaShots has compiled a list of a total of 11 new drugs approved by the EMA in September 2022
1. Rhythm’s Imcivree (setmelanotide) Receives EC’s Approval for the Treatment of Bardet-Biedl Syndrome
Imcivree
Active ingredient: setmelanotide Approved: September 06, 2022
Company: Rhythm Disease: Bardet-Biedl Syndrome
- The EC has approved Imcivree for the treatment of obesity and control of hunger associated with genetically confirmed BBS in adult & pediatric patients (≥6yrs.) with impairments in the MC4R pathway due to genetic diseases
- The expansion of Imcivree has led to the eligibility of Rhythm to receive an additional investment of $37.5M under the Revenue Interest Agreement with HealthCare Royalty & remains eligible to receive an additional investment amount of $25M payable upon achievement of agreed sales milestones in 2023
- Additionally, Imcivree has received a Priority Review from Health Canada for the treatment of obesity & control of hunger in BBS, biallelic POMC, PCSK1, or LEPR deficiency
Tecartus
Active ingredient: brexucabtagene autoleucel Approved: September 07, 2022
Company: Kite Disease: Acute Lymphoblastic Leukemia
- The approval was based on the P-I/II (ZUMA-3) study in adult patients aged 18yrs. with ALL whose disease is refractory or relapsed, following standard systemic therapy or HSCT
- The results showed that 71% of the evaluable patients achieved CR or CR with CRi with a median follow-up of 26.8mos., m-OS was 25.4mos. and 47mos. for responders (patients who achieved CR or CRi) & m-DoR was 18.6mos. in an efficacy-evaluable patients
- The safety results were consistent with the known safety profile for Tecartus while grade ≥3 CRS and neurologic adverse reactions were reported in 25% and 32% of patients, and the therapy was generally well managed. The therapy marks the first approved treatment option for patients
LTX-315
Active ingredient: brexucabtagene autoleucel Approved: September 07, 2022
Company: Lytix Biopharma Disease: Advanced Melanoma
- The EC has granted approval to initiate the P-II (ATLAS-IT-05) study in 3 EU countries to evaluate LTX-315 + pembrolizumab for advanced melanoma. The study is currently ongoing in the US, initiated in 2021 at MD Anderson Cancer Center. Patients’ enrolment is ongoing & is expected to be completed in early 2023
- The study purposes are to determine the effectiveness of LTX-315 + pembrolizumab in inducing responses for patients who have failed prior anti-PD 1/PD L1 immune checkpoint therapy
- In 3 EU countries, the study will take place at highly recognized sites with intratumoral immunotherapy experience. The same procedures as in the US will be followed, and each site will be overseen by melanoma experts
Opdualag
Active ingredient: nivolumab and relatlimab Approved: September 19, 2022
Company: BMS Disease: Unresectable or Metastatic Melanoma
- The approval was based upon an exploratory analysis from the P-II/III (RELATIVITY-047) evaluating the fixed-dose combination of nivolumab (480mg) + relatlimab (160mg) vs nivolumab (480mg) alone in a ratio (1:1) in 714 patients aged 12yrs. with prior untreated metastatic or unresectable melanoma with tumor cell expression <1% across EU member states, Iceland, Liechtenstein & Norway
- The results showed an m-PFS (6.7mos. vs 3.0mos.), m-OS has not yet been reached, and the incidence of grade 3-5 adverse reactions was 43% vs 35%. The trial also met its 1EPs of PFS in the all-comer population & no new safety events were observed
- Opdualag is 1st approved LAG-3-blocking Ab combination for advanced melanoma in the EU
Evusheld
Active ingredient: tixagevimab and cilgavimab Approved: September 20, 2022
Company: AstraZeneca Disease: COVID-19
- The EC has approved Evusheld in adults & adolescents aged ≥12yrs. with COVID‑19 who do not require supplemental oxygen & with a high risk of progressing to sev. COVID‑19
- The approval was based on the P-III (TACKLE) trial evaluating Evusheld (300mg each, IM) vs PBO in a ratio (1:1) in 903 patients with COVID-19 who were symptomatic for ≤7 days
- The results showed that 1 IM dose of Evusheld provides protection against progression to sev. COVID-19 or death from any cause, 90% were at high risk due to co-morbidities or age, 50% reduction in relative risk of progressing to sev. COVID-19 or death @29 Days in non-hospitalized patients; risk reduction (88% & 67%) within 3 & 5 days of symptom onset in pre-specified analyses, was well tolerated
Nulibry
Active ingredient: fosdenopterin Approved: September 20, 2022
Company: Zydus Lifesciences Disease: Molybdenum Cofactor Deficiency Type A
- The EC has granted marketing authorization for Nulibry to treat MoCD Type A. The EC decision was based on the efficacy & safety data of the Nulibry vs natural history study
- The EC’s centralized marketing authorization will be valid in all EU member states, Iceland, Liechtenstein, and Norway. The regulatory filing to the MHRA is expected in the coming months
- Nulibry is a cPMP substrate replacement therapy & was approved in the US in 2021 to reduce the risk of mortality in patients with MoCD Type A. In Mar 2022, Sentynl holds the global rights to Nulibry and is responsible for the ongoing development and commercialization in the US and also for developing, manufacturing, and commercializing fosdenopterin globally
Vabysmo
Active ingredient: faricimab Approved: September 20, 2022
Company: Roche Disease: Diabetic Macular Edema
- The approval was based on the 4 P-III studies (TENAYA & LUCERNE) in nAMD at 1yr. & (YOSEMITE & RHINE) in DME at ~2yr. evaluating Vabysmo vs aflibercept in 3220 patients
- All studies met their 1EPs i.e., patients treated with Vabysmo administered at q4mos. achieved similar vision gains & anatomical improvements vs aflibercept (q2mos.). The 2EPs of (TENAYA & LUCERNE) and (YOSEMITE & RHINE) studies, (46% & 45% and 52% & 51%) were able to be treated q4mos. in 1yr. and (34% & 33% and 21% & 20%) at q3mos.
- In (YOSEMITE & RHINE) study @2yr., patients achieved q4mos. dosing increased to 60% & 64% while 18% & 14% at q3mos., was well tolerated with a favorable benefit-risk profile, 33% & 21% achieved fewer median no. of inj. for nAMD & DME
8. Aurinia’s Lupkynis (voclosporin) Receives EC’s Approval for the Treatment of Lupus Nephritis
Lupkynis
Active ingredient: voclosporin Approved: September 20, 2022
Company: Aurinia Disease: Lupus Nephritis
- The EC has granted marketing authorization for Lupkynis in adults with active LN. The MHRA’s decision in Great Britain is expected in the coming weeks
- The EC’s decision was based on the P-III (AURORA 1 & 2) study to evaluate Lupkynis which showed that voclosporin + MMF & low-dose corticosteroids led to superior complete renal response rates @52wks. over MMF and low-dose corticosteroids alone. The safety profile was comparable to MMF & low-dose corticosteroids alone
- The centralized marketing authorization is valid in EU member states, Iceland, Liechtenstein, Norway & Northern Ireland. Under the agreement with Otsuka, Aurinia will receive a $30.0M EC approval-related milestones & is eligible to receive a regulatory & reimbursement milestone along with royalties
Nulibry
Active ingredient: fosdenopterin Approved: September 21, 2022
Company: BridgeBio Pharma Disease: MoCD Type A
- The EC has granted marketing authorization for Nulibry as 1st therapy for the treatment of MoCD Type A. The therapy will be available to qualified patients through an early access program
- The EC’s decision was based on the efficacy and safety data from the three clinical trials to evaluate the efficacy of Nulibry vs natural history study which showed that patients achieved a 7.1 times lower risk of death in the genotype-matched analysis with 86% vs 52% surviving @3yrs.
- The EC's centralized marketing authorization is valid in all EU member states, Iceland, Liechtenstein, & Norway. A regulatory filing to the MHRA is expected in the coming months as part of the EC decision reliance procedure
Amvuttra
Active ingredient: vutrisiran Approved: September 21, 2022
Company: Alnylam Disease: Hereditary Transthyretin-mediated Amyloidosis
- The EC has granted marketing authorization for Amvuttra to treat hATTR amyloidosis in adult patients with stage 1 or 2 polyneuropathy
- The approval was based on the 18mos. results from the P-III (HELIOS-A) study evaluating Amvuttra. The trial met its 1EPs & 2EPs at 9 & 18mos. which showed improvement in signs & symptoms of hATTR amyloidosis with ≥50% of patients experiencing halting or reversal of polyneuropathy manifestations along with an encouraging safety & tolerability profile, improvement in mean change from baseline in mNIS+7 @18mos. over PBO from the P-III (APOLLO) study of patisiran
- The therapy has received ODD in the EU & US for ATTR amyloidosis & in Japan for transthyretin type familial amyloidosis with polyneuropathy
Ultomiris
Active ingredient: ravulizumab Approved: September 26, 2022
Company: AstraZeneca Disease: Generalised Myasthenia Gravis
- The EC has approved Ultomiris as an add-on to standard therapy for gMG in adult patients who are AChR Ab+
- The EC’s decision was based on the P-III (CHAMPION-MG) trial evaluating Ultomiris in a ratio (1:1) in 175 adult patients with gMG across North America, EU, Asia-Pacific & Japan for 26wks. The results showed that the therapy was superior to PBO in the 1EPs of change from baseline in MG-ADL total score @26wk. clinical benefits were seen @60wks. in prolonged follow-up results from OLE
- Reduction in treatment burden with dosing q8w & the results were published in NEJM Evidence, improvement in daily activities in patients with milder symptoms. The safety profiles were consistent with P-III trials in PNH & aHUS
Related Post: Insights+: EMA Marketing Authorization of New Drugs in August 2022
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














