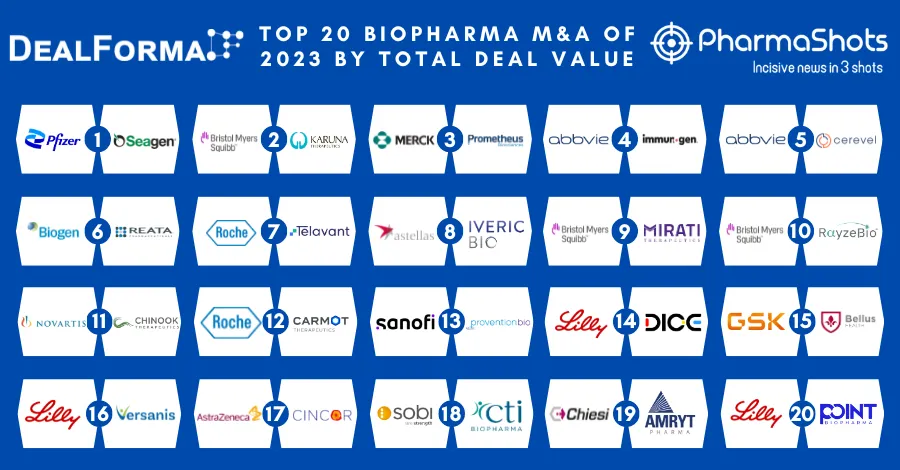
Amryt Receives CHMP Positive Opinion of Filsuvez for the Treatment of Dystrophic and Junctional EB
Shots:
- The EMA’s CHMP has adopted a positive opinion recommending the approval of Filsuvez in the EU for the treatment of partial-thickness wounds associated with dystrophic and junctional EB in patients aged ≥6mos. The product is expected to be available shortly in the EU
- The CHMP positive opinion was based on the P-III (EASE) trial in a ratio (1:1) in 223 patients including 156 pediatric patients with EB across 58 sites in 28 countries
- The EC’s decision on Filsuvez application is expected within 67 days while the MHRA is also expected to grant authorization within the same period. The centralized marketing authorization will be valid in all EU Member States, Iceland, Liechtenstein & Norway
Ref: Amryt | Image: Amryt
Click here to read the full press release

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.