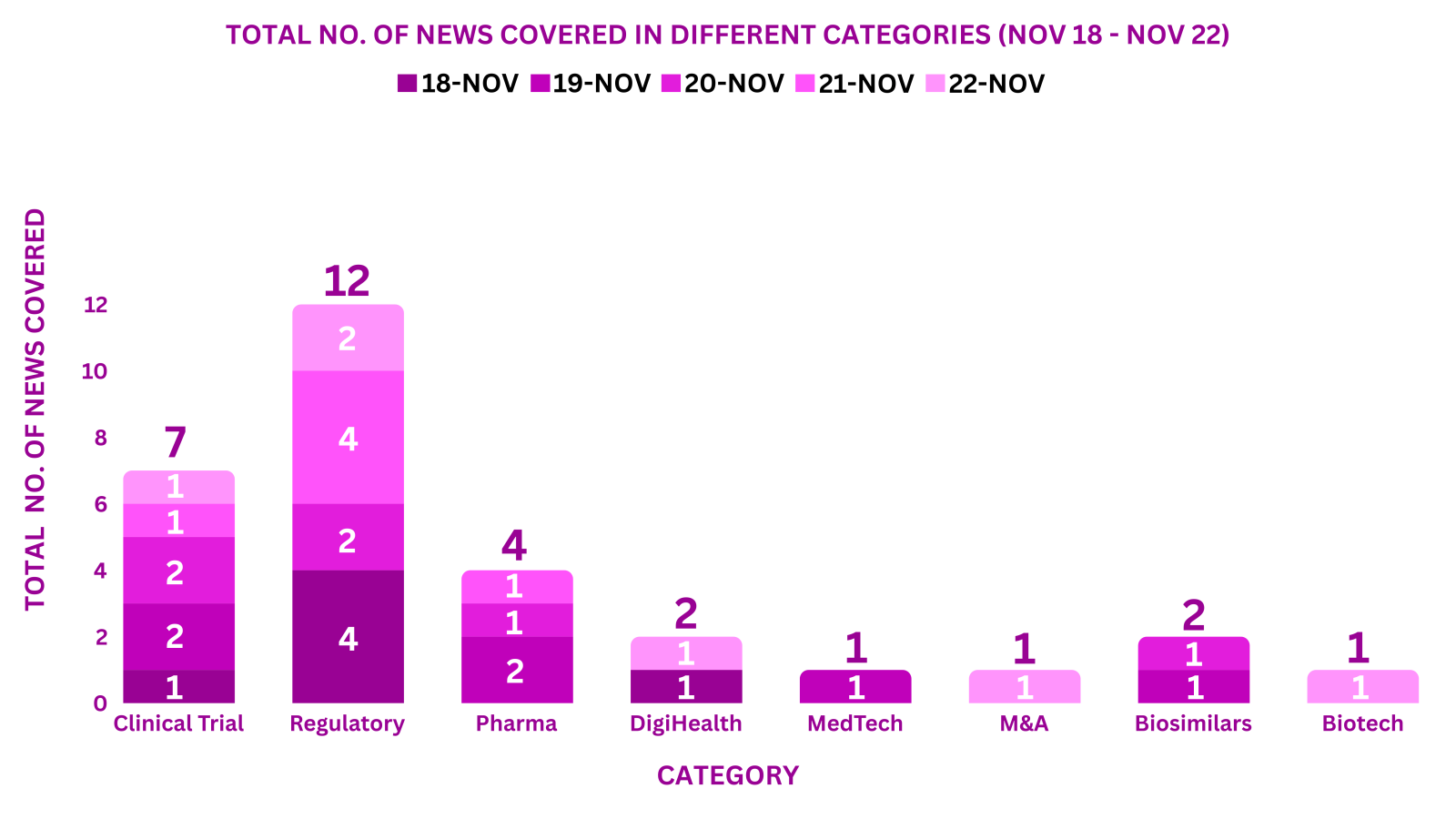
PharmaShots Weekly Snapshots (November 18 – November 22, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, DigiHealth, MedTech, M&A, Biosimilars & Biotech. Check out our full report below:

EnteroBiotix Concludes Recruitment for P-II Trial of EBX-102-02 in IBS
Read More: EnteroBiotix
Eli Lilly Reports the Data from P-II Trial of Muvalaplin in Adults with High Risk for Cardiovascular Events
Read More: Eli Lilly
GSK Reports the P-III (GLISTEN) Study Results of Linerixibat to Treat Primary Biliary Cholangitis (PBC) Patients with Cholestatic Pruritus
Read More: GSK
Merck Reports the Data from P-III Study Assessing Subcutaneous Keytruda with Berahyaluronidase Alfa to Treat Metastatic NSCLC
Read More: Merck
Novo Nordisk Highlights the Part 1 Results from P-III (ESSENCE) Study of Semaglutide to Treat MASH at AASLD 2024 - The Liver Meeting
Read More: Novo Nordisk
Endeavor BioMedicines Reports the First Patient Dosing Under P-IIb (WHISTLE-PF) Study of Taladegib (ENV-101) to Treat Idiopathic Pulmonary Fibrosis
Read More: Endeavor BioMedicines
Puma Biotechnology Commences the P-II (ALISCA-Breast1) Study of Alisertib in HR-Positive, HER2-Negative Metastatic Breast Cancer
Read More: Puma Biotechnology

Merck Reports the CHMP’s Positive Opinion of Keytruda Plus CT as a 1L Treatment of Unresectable Non-Epithelioid Malignant Pleural Mesothelioma (MPM)
Read More: Merck
BMS’ Opdivo + Yervoy Receives the CHMP’s Positive Opinion as a 1L Treatment of Metastatic Colorectal Cancer
Read More: BMS
AbbVie’s Elahere (Mirvetuximab Soravtansine) Receives the EC’s Approval to Treat Platinum-Resistant Ovarian Cancer
Read More: AbbVie
AstraZeneca Reports the CHMP’s Positive Opinion of Tagrisso (Osimertinib) for Treating Unresectable EGFR-Mutated Lung Cancer
Read More: AstraZeneca
Pfizer Reports the EC’s Approval of Hympavzi (Marstacimab) to Treat Hemophilia A/B without Inhibitors in Adults and Adolescents
Read More: Pfizer
Sangamo Therapeutics Receives the US FDA’s IND Approval for ST-503 to Treat Idiopathic Small Fiber Neuropathy
Read More: Sangamo Therapeutics
Jazz Pharmaceuticals’ Ziihera (Zanidatamab-hrii) Secures the US FDA’s Accelerated Approval to Treat HER2-Positive (IHC 3+) Biliary Tract Cancer (BTC)
Read More: Jazz Pharmaceuticals
UCB’s Bimzelx (Bimekizumab-bkzx) Secures the US FDA’s Approval for Treating Moderate to Severe Hidradenitis Suppurativa (HS)
Read More: UCB
Nurix Therapeutics’ NX-5948 Secures the EMA’s PRIME Designation for Treating R/R Chronic Lymphocytic Leukemia
Read More: Nurix Therapeutics
InxMed’s Ifebemtinib Secures the NMPA’s Breakthrough Therapy Designation as a 1L Treatment of KRAS G12C Mutated NSCLC
Read More: InxMed
GSK’s Arexvy Vaccine Receives the MHLW’s Approval for its Expanded Age Indication to Prevent RSV Disease
Read More: GSK
Bayer Reports the US FDA’s sNDA Acceptance of Nubeqa (Darolutamide) to Treat Metastatic Hormone-Sensitive Prostate Cancer
Read More: Bayer

Ratio Therapeutics Partners with Novartis to Advance SSTR2-Targeting Radiotherapeutic Candidate for Cancer
Read More: Ratio Therapeutics and Novartis
Bayer Join Forces with Cytokinetics to Develop and Commercialize Aficamten in Japan
Read More: Bayer and Cytokinetics
Pharmanovia Collaborates with Lindis Biotech to Commercialize Catumaxomab for Treating Malignant Ascites
Read More: Pharmanovia and Lindis Biotech
Alloy Therapeutics Inks a Pact with Takeda to Develop Cell Therapy Platform
Read More: Alloy Therapeutics and Takeda

BrightHeart Receives the US FDA Approval for its AI Software Transforming Prenatal Fetal Heart Ultrasound Evaluations
Read More: BrightHeart
Fresenius Kabi Reports the Reguatory Submission to the US FDA for the Aurora Xi Plasmapheresis System Software Version 2.0 with New Nomogram
Read More: Fresenius Kabi

Abbott Reports Late-Breaking Data of Amplatzer Amulet Device for Treating Patients with Atrial Fibrillation at Risk of Stroke
Read More: Abbott

Novartis Reports the Acquisition of Kate Therapeutics for ~$1.1B
Read More: Novartis and Kate Therapeutics

Samsung Bioepis and Biogen Report the EC’s Approval of Opuviz (Biosimilar, Eylea)
Read More: Samsung Bioepis and Biogen
Xbrane Biopharma and Intas Pharmaceuticals Join Forces to Develop Biosimilar of Opdivo (Nivolumab)
Read More: Xbrane Biopharma and Intas Pharmaceuticals

Xenetic Biosciences Reports Preclinical Data of Potential Co-Administration of DNase I with CAR T Cells for Melanoma Lung Metastasis
Read More: Xenetic Biosciences
Related Post: PharmaShots Weekly Snapshots (November 11 – November 15, 2024)
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.