
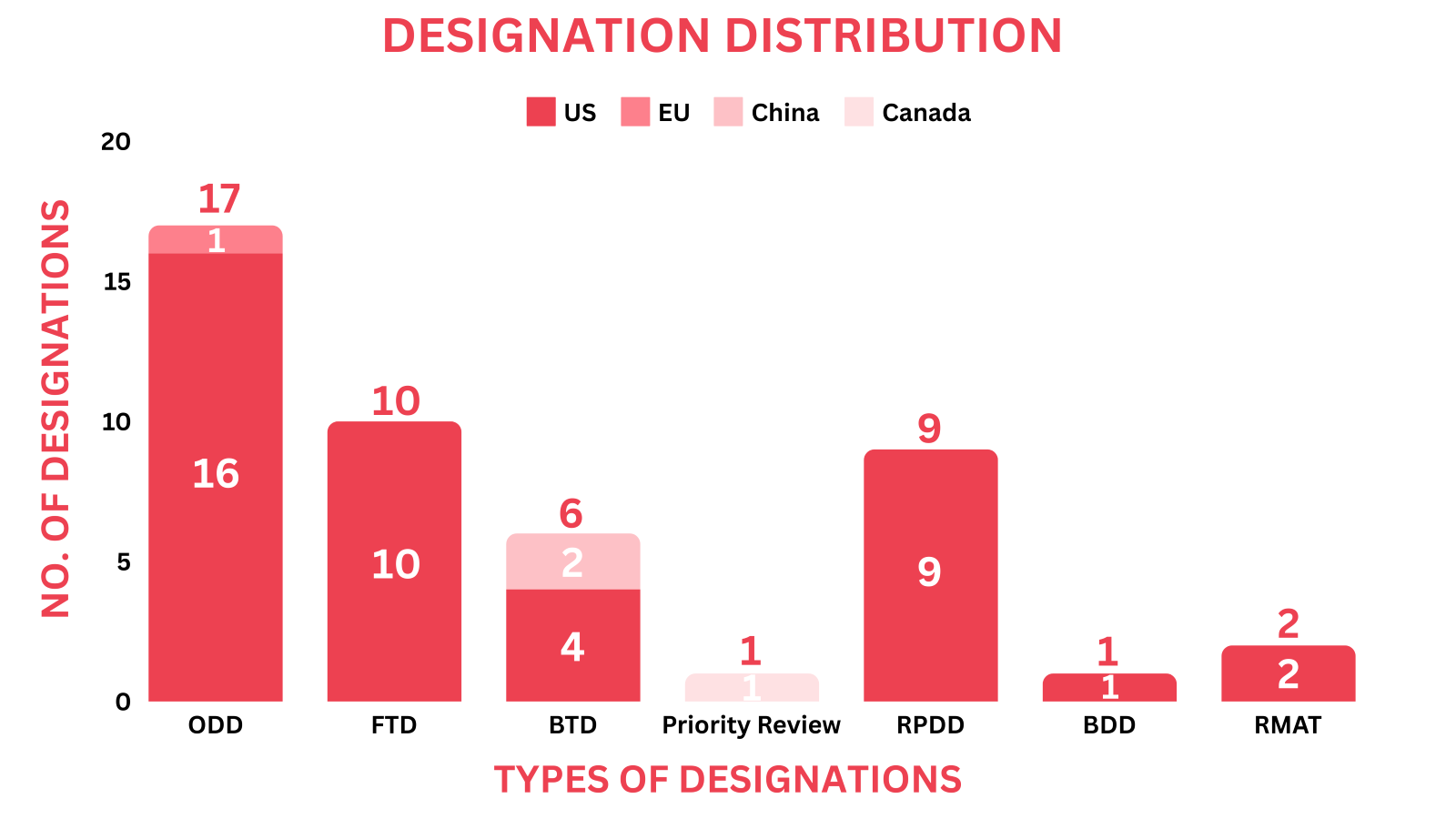
New Drug Designations - September 2024
Shots:
- PharmaShots' designation report provides a concise overview of several drugs and their designations by the FDA, NMPA, Health Canada and EMA
- The September 2024 report covers regulatory designations for 42 drugs and 1 device, including 16 small molecules, 6 biologics, 12 cell and gene therapies, and 1 device, among others
- Significant trends this month show, Longboard’s Bexicaserin received the FDA’s ODD & RPDD for Dravet Syndrome and YolTech’s YOLT-203 was also designated with the FDA’s ODD & RPDD for Primary Hyperoxaluria Type

Yorvipath
.png)
- The US FDA granted Orphan Drug Designation (ODD) to Yorvipath (palopegteriparatide), also known as TransCon PTH, for treating adult hypoparathyroidism.
- Yorvipath is designed to enable continuous PTH release, providing effective hormone replacement for adults with hypoparathyroidism
Bexicaserin
.png)
- Bexicaserin has received Orphan Drug Designation and Rare Pediatric Disease Designation (RPDD) from the FDA for treating Dravet syndrome
- Bexicaserin is under a global P-III (DEEp SEA) trial as part of the DEEp program (DEEp SEA, DEEp OCEAN & DEEp OLE) that will enroll ~480 subjects across 80 sites globally, with its launch planned in Q4’28
- Additionally, Longboard Pharmaceuticals is set to be acquired by Lundbeck to strengthen their neuro-rare disease portfolio
Certepetide
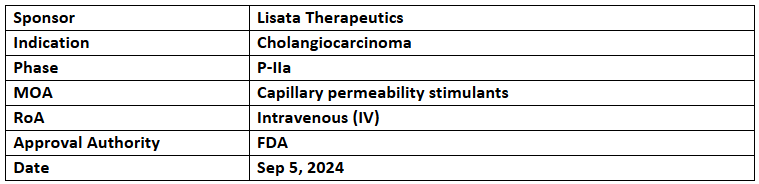
- The US FDA has granted orphan drug designation to the company’s Certepetide for treating Cholangiocarcinoma
- Certepetide is being assessed under the P-IIa clinical evaluation for its preliminary efficacy, safety and tolerability in addition to SoC vs PBO + SoC alone as a 1L and 2L treatment of cholangiocarcinoma
- Certepetide activates the C-end rule transport mechanism for effective tumor-specific delivery of co-administered anti-cancer drugs. It also reduces immunosuppression and inhibit metastasis by modifying the tumor microenvironment
AISA-021 (Cilnidipine)
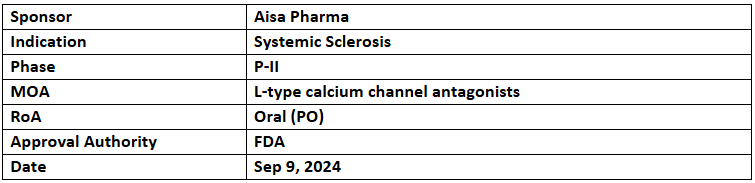
- The US FDA has granted ODD to AISA-021 (Cilnidipine) for treating Systemic Sclerosis (SSc)
- Aisa's P-II study showed improved symptoms of SSc, including disease severity, pain, gastrointestinal dysfunction, skin ulcers, disability & Raynaud’s symptoms
- NIH's initial screening of Cilnidipine for the Preclinical Pain Screening Platform did not find its abuse liability or addictive potential. Additional results are pending
- The company will also highlight following posters at ACR 2024:
- ‘AISA 021, a Novel Calcium Channel Antagonist in Development for Raynaud’s & Systemic Sclerosis, Has Activity at Sodium Channel Targets for Pain Relief and Treats Scleroderma Pain Better Than Current Calcium Channel Blockers in a Phase 2A Study’
- ‘Preliminary Results from the RECONNOITER Trial, a Phase 2 Study of AISA 021 in the Treatment of Secondary Raynaud’s, Primarily Due to Systemic Sclerosis’
Elraglusib
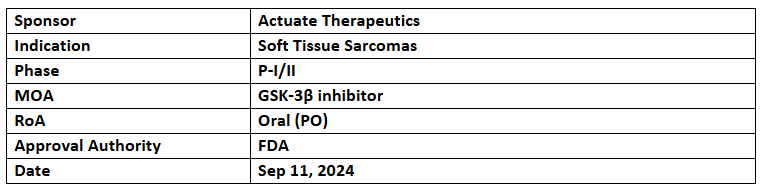
- The US FDA has granted ODD to Elraglusib for treating soft tissue sarcomas
- Elraglusib is a GSK-3β inhibitor which showed good safety & antitumor activity in various solid tumors such as melanoma, Ewing sarcoma, colorectal & pancreatic cancers
Tebapivat (AG-946)
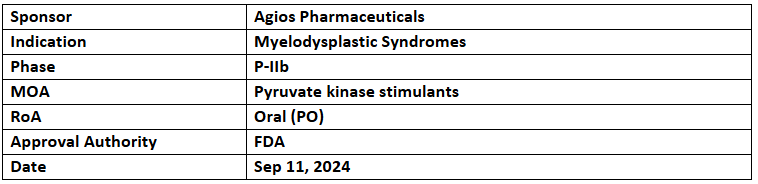
- Tebapivat, a pyruvate kinase activator, has been granted Orphan Drug Designation by the US FDA for the treatment of myelodysplastic syndromes (MDS).
- The company has completed its P-IIa trial for lower-risk MDS and is currently conducting a Phase IIb trial for the same indication.
ATSN-201

- ATSN-201 is being evaluated in the P-I/II (LIGHTHOUSE) trial, which includes dose escalation and expansion phases, to treat XLRS resulting from RS1 gene mutations in male patients aged 6 years and older. Recruitment is currently ongoing.
- ATSN-201 is a gene therapy utilizing AAV.SPR, a novel spreading capsid designed for therapeutic gene expression in central retinal photoreceptors while minimizing the risk of foveal detachment.
AMT-191
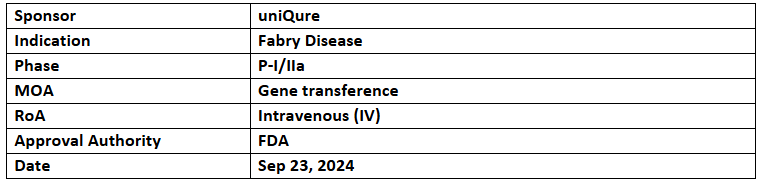
- AMT-191 is being assessed in P-I/IIa trial for its safety, tolerability & early efficacy in two cohorts (low dose: 6x10^13gc/kg or a high dose: 3x10^14gc/kg) of up to 6 adult male patients across the US. Patients will continue their regular enzyme replacement therapy for 24mos.
- The first patient has been dosed in the trial. The company will generate clinical PoC data, with initial results expected in 2025
- AMT-191 is an investigational gene therapy administered as a single intravenous dose. It uses an AAV5 vector with a proprietary, highly potent promoter to deliver the GLA transgene, specifically targeting the liver to produce the GLA protein.
LMP744

- Amarex & Gibson Oncology have received the US FDA’s ODD for LMP744 to treat Glioblastoma. LMP744 crosses the blood-brain barrier at 10 times the cancer-killing concentration and maintains high levels for over 24hrs. per dose
- LMP744 was discovered under a multi-year collaboration with cMyc expert, Dr. Danzhou Yang of Purdue University and was found to target both TOPO 1 and cMyc oncogene. LMP400 was also identified along with LMP744
- LMP744 and LMP400 are set to enter a P-II study for Recurrent Gliomas in collaboration with the NIH
Navenibart (STAR-0215)

- The US FDA has granted orphan drug designation to the company’s Navenibart (STAR-0215) for the treatment of Hereditary Angioedema (HAE)
- Navenibart was being assessed under the P-Ib/II (ALPHA-STAR) study that depicted favorable safety & tolerability as well as monthly attack rates reduction by 90-96% with once or twice over 6mos. dosing. This data will form the basis of P-III study planned during Q1’25, with topline data by the YE’26
- Navenibart (STAR-0215) is a mAb that works by inhibiting kallikrein and is developed to offer long-acting, safe & effective attack prevention for HAE with Q3M/Q6M dosing
ABD-147
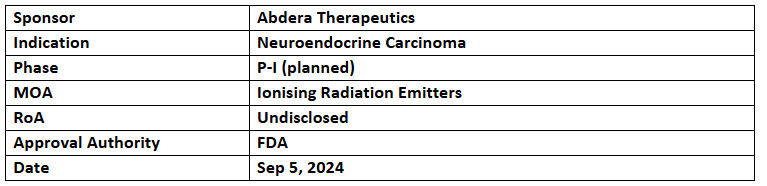
- ABD-147 has received the US FDA’s ODD for treating Neuroendocrine Carcinoma
- The company is planning to begin P-I study of ABD-147 for SCLC or large cell neuroendocrine carcinoma (LCNEC) in patients previously treated with Pt-based therapy
- ABD-147 is a precision radiopharmaceutical therapy that transfers Actinium-225 to solid tumors with DLL3 expression
CMP-CPS-001
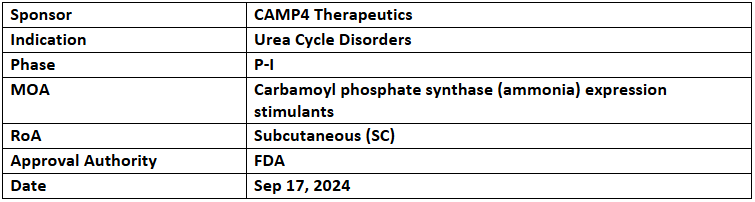
- CMP-CPS-001 is being investigated under P-I study for its safety & efficacy among healthy subjects. It has also received the FDA’s RPDD
- CMP-CPS-001 is an antisense oligonucleotide (ASO) drug that targets CPS1 to treat UCDs. It aims to increase CPS1 protein levels by binding to a regulatory RNA sequence and improving CPS1 gene expression
YOLT-203
.png)
- The US FDA has granted ODD to YOLT-203, developed using YolCas12 gene editing technology, for treating PH1
- YOLT-203 is being assessed under investigator-initiated trial with the dosing of first adult & first pediatric patient done in Aug 2024. It shown reduced oxalate levels in preclinical models
SMT-M01
.png)
- The US FDA has also granted ODD along with RPDD to SMT-M01 for treating DMD
- The company’s AlphaStem AI platform based SMT-M01 will enter into the clinical evaluation in the next 18 mos. to offer potential mutation-agnostic treatment for DMD
EYD-001
.png)
- The US FDA has granted ODD to EYD-001 (TAK1 inhibitor) for treating Systemic Sclerosis
- EydisBio's preclinical data, in collaboration with Dr. John Varga at the University of Michigan, depicted the reduction of dermal thickening & p-TAK1 expression in a Systemic Sclerosis mouse model. In patient-derived skin fibroblasts, EYD-001 reduced fibroinflammatory gene expression and blocked TGFβ-driven fibrosis
SKG1108
.png)
- SKG1108 has received the US FDA’s ODD for treating Retinitis Pigmentosa
- SKG1108 is a recombinant adeno-associated virus (rAAV) vector which transfers single-stranded DNA encoding light-activatable proteins using the novel AAV.0106 capsid directly to the retina
SBL01
.png)
- SeaBeLife's preclinical data of SBL01 showed reduction in hepatotoxicity & improved survival when combined with the currently authorized product vs the product alone in an acute liver failure model
- SBL01 is a first-in-class small molecule which targets both necroptosis and ferroptosis

MM-II
.png)
- MM-II (Large Liposomes of DPPC and DMPC) has received the US FDA’s FTD for treating osteoarthritis knee pain. Its P-III trial for the same is planned
- The P-IIb study of MM-II (3mL) vs PBO depicted greater relief from pain for over 26wks. Results were highlighted at EULAR 2024
Brepocitinib
.png)
- The company has also recruited first patients in the P-III (CLARITY) study evaluating Brepocitinib for non-anterior non-infectious uveitis, alongside the US FDA’s FTD
- The P-III (CLARITY) trial will assess Brepocitinib (45mg) vs PBO in patients (n=300) with non-anterior non-infectious uveitis. Trial includes two sub-studies with a 1EP of time to treatment failure and follows the successful P-II (NEPTUNE) study & will begin after end of P-II Meeting with the FDA
- The P-II study showed a dose-dependent treatment benefit & highest efficacy on the 1EP of Treatment Failure at 6mos. Brepocitinib (45mg) resulted in a mean 4.4-point decrease in retinal vascular leakage & 0.5-point decrease in the 15mg arm. Topline 52wks. data will be available by YE’24
- Brepocitinib is also being tested in the fully enrolled P-III (VALOR) study for Dermatomyositis, with top-line data expected in H2’25 with an NDA submission to follow if successful
IBI363
.png)
- IBI363 has received the US FDA’s FTD for treating unresectable locally advanced or metastatic melanoma (except choroidal melanoma) in patients progressed on or after at least 1L therapy, incl. PD-1/L1 inhibitor
- IBI363 is currently being investigated under P-I/II trial for its safety & efficacy in various advanced malignant tumors across the US, Australia & China
- The company reported results in melanoma patients previously treated with immunotherapy at ESMO Plenary 2024. of 37 patients receiving 1mg/kg of IBI363, 11 showed objective responses (1 CR, 10 PR), with an ORR of 29.7% & a DCR of 73%
GLM101
.png)
- The US FDA has granted FTD to the company’s GLM101 for treating Phosphomannomutase 2-congenital disorder of glycosylation (PMM2-CDG)
- The P-II (GLM101-002) trial recruited 15 patients with PMM2-CDG from the US & Spain and received GLM101 (10, 20 or 30mg/kg, for up to 24 wks.). Study showed that the drug was well-tolerated without any SAEs reported and will continue recruiting pediatric patients (≥2yrs.)
- GLM101 is a mannose-1-phosphate replacement therapy intended to transfer mannose-1-phosphate directly into cells, bypassing the PMM2 enzyme deficiency to restore pathway function
ICT01
.png)
- The US FDA has granted FTD to ICT01 in addition to Azacitidine and Venetoclax for treating AML patients (≥75yrs.) with comorbidities
- The P-I/IIa (EVICTION) study of ICT01 alone & combination for solid & hematologic tumors. As reported at ESMO 2023, the P-I cohort included 26 r/r patients (mostly with AML) did not show DLTs with doses from 200μg to 75mg, Q21D
- Based on these outcomes, a dose-optimization phase in newly diagnosed AML patients was began which recruited 29 subjects. Early data for ICT01 + azacitidine-Venetoclax depicted rapid activation and migration of γ9δ2 T cells, indicating strong target engagement
Narmafotinib
.png)
- Narmafotinib has received the US FDA’s FTD for treating advanced pancreatic cancer
- The company is assessing the drug under P-II (ACCENT) study for advanced pancreatic cancer across Australia and South Korea. The FDA also recently cleared an IND for the same
PTC518
.png)
- The 12 mos. interim results from the PIVOT-HD trial of PTC518 showed a durable, dose-dependent reduction of mutant Huntingtin protein in blood cells (43% at 10mg) with similar reductions seen in CSF P-II study
- Favorable effects were also observed in disease measurements such as total motor score and cUHDRS scale. PTC518 was found to be safe & well-tolerated, without treatment-related NfL spikes
- PTC518, discovered using PTC's splicing platform, is a small molecule that can enter the blood-brain barrier & reduce mutated Huntingtin protein production, which contributes to neuronal injury & disease progression
CB-010 & CB-012
.png)
- CB-010 has received the US FDA’s FTD for treating refractory systemic lupus erythematosus (SLE) and CB-012 for r/r acute myeloid leukemia (AML)
- CB-010 (an allogeneic anti-CD19 CAR-T cell therapy) will be investigated under P-I (GALLOP) study for treating lupus nephritis (LN) and extrarenal lupus (ERL). It will begin by the YE’24
- CB-012 (an allogeneic anti-CLL-1 CAR-T cell therapy) is being assessed under the P-I (AMpLify) study for treating r/r AML
SYNCAR-001 + STK-009
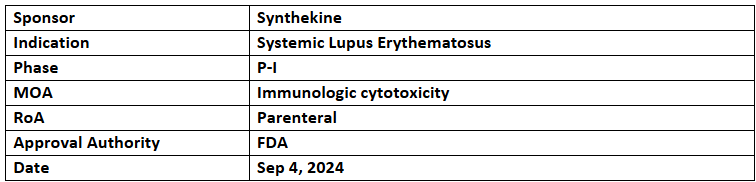
- SYNCAR-001 (CD19 CAR-T) + STK-009 (orthogonal IL-2 therapy), developed using orthoIL-2 tech, have received the US FDA’s FTD for treating severe, refractory SLE, without lymphodepletion
- The combination is being assessed under P-I (NCT05665062) study for CD19+ hematologic malignancies
- The FDA also cleared an IND for another P-I trial (NCT06544330) in non-renal SLE & lupus nephritis (LN). It will be a dose escalation study to evaluate the safety & efficacy of SYNCAR-001 + STK-009 in patients with non-renal SLE and LN. Patients will receive a single dose of SYNCAR-001 cells and weekly subcutaneous injections of STK-009 for a set duration
EO-3021
.png)
- The US FDA has granted FTD to EO-3021 (ADC) for treating advanced or metastatic G/GEJ cancer expressing Claudin 18.2 in patients progressed after prior therapy
- The designation is based on nonclinical and early clinical data from ongoing P-I study, showing ORR of 42.8%
- Elevation Oncology will advance EO-3021 alone through dose expansion, with additional data expected in early 2025 & starting the combination phase later in 2024

Sanbexin
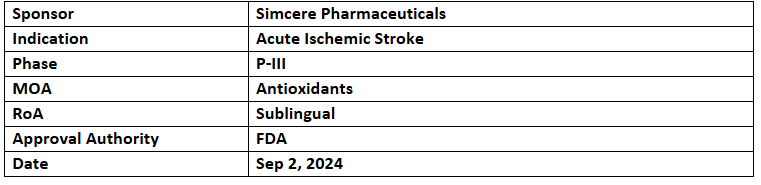
- Designation was based on the P-III study of Sanbexin vs PBO to treat acute ischemic stroke in Chinese patients. It depicted significantly enhanced neurological recovery & independent living ability, with a favorable safety profile. Data was published in JAMA Neurology
- NDA for the same was accepted by China's NMPA in Jun 2023. P-I study among healthy subjects has been completed across the US
Plozasiran
.png)
- The US FDA has granted BTD to Plozasiran as an adjunct for lower the number of triglycerides in adults with familial chylomicronemia syndrome (FCS)
- The P-III (PALISADE) trial is assessing Plozasiran (25mg & 50mg, SC, Q3M) vs PBO to treat genetically confirmed/clinically diagnosed FCS in adults (n=75). Patients could join a 2-part extension period where all receive Plozasiran post completing randomization
- Trial depicted a reduction in triglycerides by 80% & decreased risk of acute pancreatitis by 83%
- Arrowhead plans NDA submission to the US FDA by the YE’24, with additional global authorities afterward
Infigratinib
.png)
- The US FDA has granted BTD to Infigratinib for treating children with Achondroplasia. Once approved, the company will be eligible for priority review voucher
- Designation was based on P-III (PROPEL 3) study assessing Infigratinib to treat children & adolescents with achondroplasia, for which the recruitment will be completed by the YE’24
- Cohort 5 of the study depicted a significant & sustained increase in AHV of +2.51cm/yr at 12mos. & +2.50cm/yr at 18mos.; improved body proportionality was also observed at 18 mos.
- BridgeBio is also exploring Infigratinib's potential in Hypochondroplasia and other Skeletal Dysplasias with unmet needs
NTX-001
.png)
- The US FDA has granted BTD to NTX-001 for treating peripheral nerve injury requiring repair, based on the P-II (NEUROFUSE) study
- The company anticipates a P-III pivotal study for NTX-001 in early 2025 based on favorable 24wks. results from the P-II trial. Complete 48wks. data will be highlighted at future conferences
- The P-III study will assess NTX-001's efficacy & safety vs PBO in patients with peripheral nerve injury after final ongoing protocol discussion with the FDA
Blenrep (balintawak mafodotin)
.png)
- The NMPA has granted BTD to the combination of Blenrep (belantamab mafodotin) and bortezomib + dexamethasone (BorDex) for treating r/r multiple myeloma, supported by the P-III (DREAMM-7) study
- The P-III head-to-head (DREAMM-7) study assessing the safety & efficacy of Blenrep (2.5mg/kg, IV, Q3W) + BorDex vs daratumumab + BorDex to treat r/r MM patients (n=494) treated with at least 1L of therapy
- Study reached its 1EP, showing improved PFS; an OS trend was established during interim analysis which was insignificant, though its evaluation continues. All 2EPs were also improved including deeper and more durable responses
SC0062
.png)
- The NMPA has granted BTD to SC0062 for IgAN with proteinuria supported by the data from P-II (2-SUCCEED) study assessing its safety & efficacy to treat two types of CKD, IgA nephropathy & diabetic kidney disease
- Study showed significantly reduced proteinuria with a clear dose-response & good safety as well as low rates of peripheral edema; when combined with SGLT2 inhibitors, SC0062 showed a favorable safety profile. Results from the ongoing DKD cohort are expected in Q4’24
- The company also anticipates a P-III clinical evaluation of SC0062 for IgAN, DKD & other CKD diseases across the globe, incl. China

Omaveloxolone
.png)
- The Health Canada has accepted and granted priority review to the NDS of Omaveloxolone for treating Friedreich’s ataxia in adults & adolescents (≥16yrs.), with the decision expected in early 2025
- NDS submission was based on the part 2 (MOXIe) study assessing the safety & efficacy of Omaveloxolone vs PBO, showing significant improvement in the modified Friedreich Ataxia Rating Scale (mFARS) scores at 48wks.

Bexicaserin
.png)
- Bexicaserin has also received the US FDA’s ODD along with RPDD for Dravet syndrome
- It is under a global P-III (DEEp SEA) trial as part of the DEEp program (DEEp SEA, DEEp OCEAN & DEEp OLE) that will enroll ~480 subjects across 80 sites globally, with its launch planned in Q4’28
- Additionally, Lundbeck has agreed to acquire Longboard under a strategic agreement to strengthen its neuro-rare disease portfolio
Descartes-08
.png)
- The US FDA has granted RPDD to Descartes-08 for treating juvenile dermatomyositis (JDM). Cartesian will be eligible for a priority review voucher, if approved
- Descartes-08 is currently being assessed under P-IIb study for generalized myasthenia gravis, another P-II study for systematic lupus erythematosus as well as a P-II basket study for additional autoimmune disorders. IND for the basket study is anticipated in the YE’24 which will include juvenile SLE, juvenile MG & other conditions
- Descartes-08 is an autologous mRNA CAR T-cell therapy targeting BCMA that requires no preconditioning CT and avoids the risk of genomic integration linked to cancer formation
NS-050/NCNP-03
.png)
- The US FDA has granted RPDD to NS-050/NCNP-03 for treating DMD. It was jointly discovered by the National Center of Neurology and Psychiatry (NCNP) and Nippon Shinyaku
- The company is planning a P-I/II trial to assess its safety & effectiveness for treating DMD across the US & Japan
- NS-050/NCNP-03 misses out a part of the dystrophin gene to generate a shorter length functional protein, aiming to slow muscle deterioration
ETD001
.png)
- ETD001 is currently under P-IIa study for its safety & efficacy to treat CF and generate proof-of-concept; lung function (FEV1) in patients not receiving CFTR modulator therapy will be evaluated. Dosing of the first patient has been done in Jul 2024 and is anticipated to conclude in 2025
- ETD001 showed a well-tolerated profile in healthy subjects during a P-I study and demonstrated long-acting effects in non-clinical studies
BPM31510
.png)
- The UD FDA has granted RPDD to BPM31510 for the treatment of primary coenzyme Q10 deficiency, which makes the company eligible to receive priority review voucher
- BPM31510 has also received ODD for glioblastoma multiforme (GBM) & pancreatic cancer while its topical form has been granted with ODD for epidermolysis bullosa (EB)
LP-184
.png)
- The US FDA has granted RPDD to LP-184 for 3 rare children’s cancers namely, malignant rhabdoid tumors, rhabdomyosarcoma & hepatoblastoma. The company is also eligible to receive priority review voucher
- LP-184 is under a P-IA study that will recruit 50-60 patients with various solid tumors. Lantern will plan future studies for pediatric cancers like ATRT, MRT, RMS, and hepatoblastoma based on these study results
- LP-184 has shown tumor regression & extended EFS in specialized models from the Pediatric Preclinical Testing Program (PPTP), supported by the NCI to identify promising childhood cancer therapies
YOLT-203
.png)
- The US FDA has granted RPDD to YOLT-203, developed using YolCas12 gene editing technology, for treating PH1
- YOLT-203 is being assessed under investigator-initiated trial with the dosing of first adult & first pediatric patient done in Aug 2024. It shown reduced oxalate levels in preclinical models
SMT-M01
.png)
- The US FDA has also granted ODD along with RPDD to SMT-M01 for treating DMD
- The company’s AlphaStem AI platform based SMT-M01 will enter into the clinical evaluation in the next 18 mos. to offer potential mutation-agnostic treatment for DMD
MDL-101
.png)
- The US FDA has granted RPDD to MDL-101 for treating congenital muscular dystrophy type 1a (LAMA2-CMD). On approval, the company will be eligible for priority review voucher for another product
- MDL-101 is a gene therapy targeting the LAMA1 gene, using a muscle-specific AAV vector. It upregulates LAMA1 in muscle tissue to compensate for the function loss due to LAMA2 mutations

Molecular Culture ID Technology
.png)
- The company’s Molecular Culture ID tech has received the US FDA’s BDD for the diagnosis of bacterial infections
- It is a diagnostic test that uses advanced chemistry & AI to identify ~200 bacterial species & enables accurate results on the same day, improving patient outcomes, reducing costs & enhancing the diagnosis of infections like pleural, joint & wound infections
- Inbiome aims the US launch of the test by early 2026 and is collaborating with the hospitals on implementation studies

BBP-812
.png)
- The US FDA has granted RMAT designation to BBP-812 (AAV9 gene therapy) for Canavan disease, based on the 12mos. data from first 8 patients enrolled in P-I/II (CANaspire) study
- The P-I/II (CANaspire) trial assesses the safety, tolerability & PD of BBP-812 (IV) among pediatric patients with Canavan disease. 1EP is change in urine & CNS NAA levels from baseline along with motor function & development evaluation
- Results, to data, showed functional improvements in key parameters like head control, sitting upright, reaching for & grasping objects & visual tracking along with reduced NAA levels in both urine & CNS linked to mild disease. It was well-tolerated & had a safety profile aligning with other AAV9 gene therapies
P-BCMA-ALLO1
.png)
- The RMAT designation was based on results from P-I trial depicting favorable effectiveness, safety profile & rapid 'off-the-shelf' patient access
- P-BCMA-ALLO1 is a stem cell memory T cell (TSCM)-based allogeneic CAR-T cell therapy currently being investigated under P-I/Ib trial for treating r/r multiple myeloma
- The company recently presented data from P-I portion at the IMS 2024, with additional data expected in late 2024, pending coordination with Roche
References
- Ascendis Pharma
- Longboard Pharmaceuticals
- Lisata Therapeutics
- Aisa Pharma
- GlobeNewswire
- Agios Pharmaceuticals
- Atsena Therapeutics
- uniQure
- BusinessWire
- Astria Therapeutics
- Abdera Therapeutics
- CAMP4 Therapeutics
- YolTech Therapeutics
- Skyline Therapeutics
- SeaBeLife
- Sun Pharma
- PRNewswire
- Innovent Biologics
- ImCheck Therapeutics
- Amplia Therapeutics
- PTC Therapeutics
- Caribou Biosciences
- Synthekine
- Elevation Oncology
- Simcere Pharmaceuticals
- Arrowhead Pharmaceuticals
- BridgeBio Pharma
- GSK
- BioCity Biopharma
- Biogen
- Cartesian Therapeutics
- NS Pharma
- Enterprise Therapeutics
- BPGbio
- Lantern Pharma
- Modalis Therapeutics
- Inbiome
- Poseida Therapeutics
Related Post: New Drug Designations - August 2024
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














