
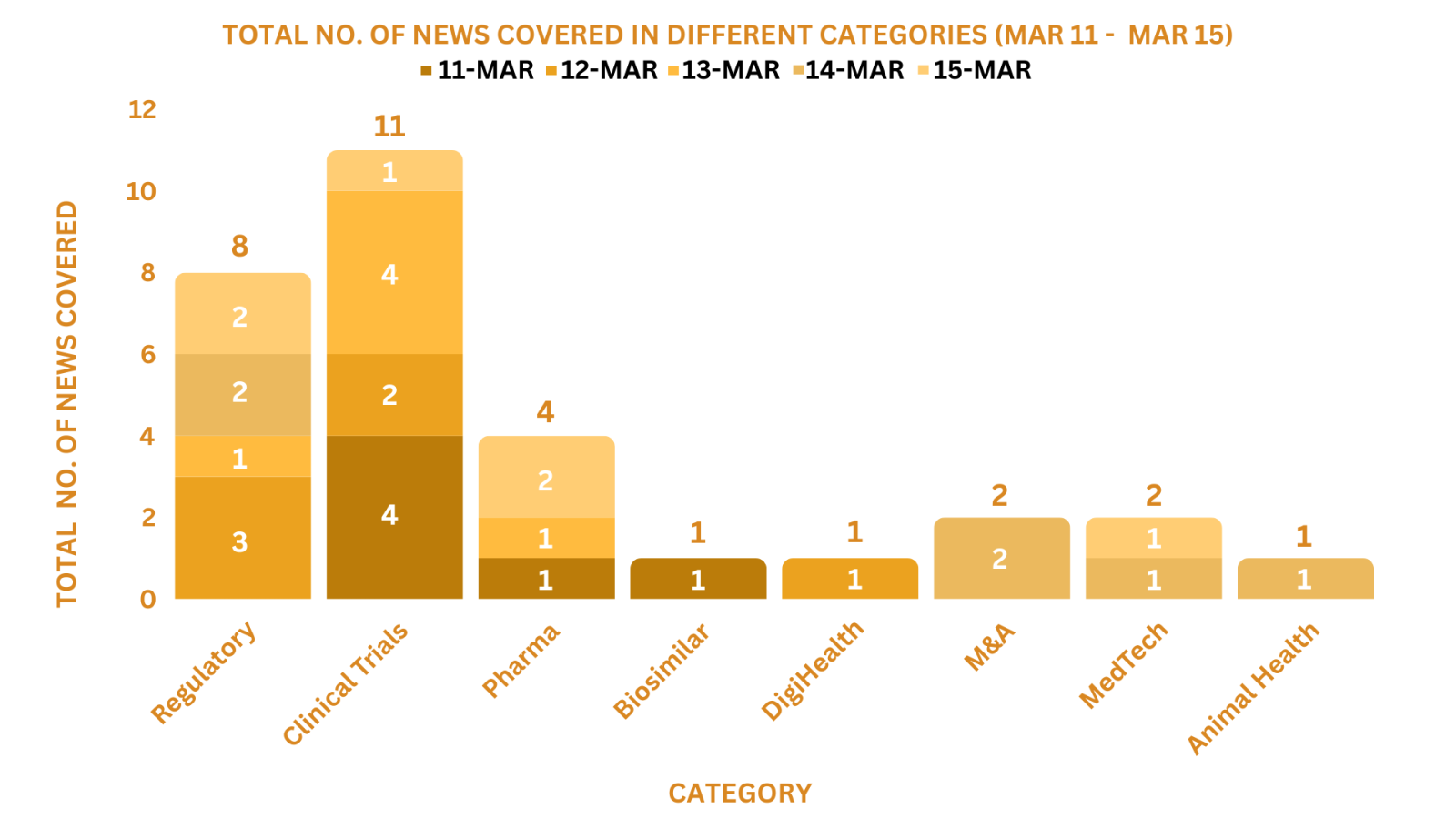
PharmaShots Weekly Snapshots (March 11 – March 15, 2024)
This week PharmaShots’ news was all about the updates on Regulatory, Clinical Trials, M&As, Pharma, Digital Health, Animal Health & MedTech. Check out our full report below:

Johnson & Johnson Submits sBLA of Tremfya to the US FDA for the Treatment of Ulcerative Colitis
Read More: Johnson & Johnson
Travere Therapeutics Submits sNDA of Filspari (sparsentan) to the US FDA to Treat IgA Nephropathy (IgAN)
Read More: Travere Therapeutics
The US FDA Grants Orphan Drug Designation to Terns Pharmaceuticals’ TERN-701 for Treating Chronic Myeloid Leukemia
Read More: Terns Pharmaceuticals
InnoCare Reports Approval to Initiate Clinical Study of ICP-248 in Combination with Orelabrutinib for Treating CLL/SLL in China
Read More: InnoCare
The EC Approves Pfizer’s 20-Valent Pneumococcal Conjugate Vaccine for the Protection Against Pneumococcal Disease in Pediatric Population
Read More: Pfizer
Daiichi Sankyo Reports NDA Submission of Datopotamab Deruxtecan to the MHLW for Treating Advanced Breast Cancer
Read More: Daiichi Sankyo
The US FDA Grants Accelerated Approval to BMS’ Breyanzi for Treating R/R Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL)
Read More: BMS
BeiGene’s Tevimbra Gains the US FDA’s Approval for Treating Advanced or Metastatic Esophageal Squamous Cell Carcinoma
Read More: BeiGene
Eli Lilly Highlights the P-III Trial Results of Lebrikizumab for People with Skin of Color and Moderate-to-Severe Atopic Dermatitis at American Academy of Dermatology (AAD) Annual Meeting
Read More: Eli Lilly
Incyte Features the P-II Study Data of Povorcitinib to Treat Prurigo Nodularis at AAD’24
Read More: Incyte
Sanofi Highlights Results from the P-IIb (STREAM-AD) Trial of Amlitelimab for Treating Atopic Dermatitis at AAD’24
Read More: Sanofi
Sensorion Reports the P-IIa Study Results of SENS-401 for Residual Hearing Preservation
Read More: Sensorion
Acadia Pharmaceuticals Highlights Results from the P-III (ADVANCE-2) Trial of Pimavanserin for Treating Negative Schizophrenia Symptoms
Read More: Acadia Pharmaceuticals
Larimar Therapeutics Reports First Patient Dosing with Nomlabofusp in the Open Label Extension Trial for the Treatment of Friedreich’s Ataxia
Read More: Larimar Therapeutics
Pfizer Reports Data from the P-III (ECHELON-3) Clinical Evaluation of Adcetris for the Treatment of Diffuse Large B-cell Lymphoma (DLBCL)
Read More: Pfizer
Astrocyte Pharmaceuticals’ Receives the US FDA’s IND Clearance of AST-004 for the Treatment of Acute Ischemic Stroke
Read More: Astrocyte Pharmaceuticals
89bio Reports Commencement of the P-III (ENLIGHTEN) Study Evaluating Pegozafermin for the Treatment of Metabolic Dysfunction-Associated Steatohepatitis (MASH)
Read More: 89bio
Longbio Pharma Presents the P-I Trial Results of LP-003 for the Treatment of Chronic Spontaneous Urticaria at AAD 2024
Read More: Longbio Pharma
Merck Highlights the P-III (KEYNOTE-A18) Study Results of Keytruda for Treating Cervical Cancer
Read More: Merck

Boehringer Ingelheim and Sosei Heptares Partner for the Development of Therapies Addressing Schizophrenia Symptoms
Read More: Boehringer Ingelheim & Sosei Heptares
Merck and Pearl Bio Partner for the Discovery of Novel Biological Therapies to Treat Cancer
Read More: Merck & Pearl Bio
Bayer Join Forces with Aignostics to Strengthen its Precision Oncology Portfolio
Read More: Bayer & Aignostics
Biotheus and Hansoh Pharma Expand their Collaboration to Develop EGFR/cMET Bispecific Antibody-Drug Conjugates
Read More: Biotheus & Hansoh Pharma

Celltrion Submits BLA to the US FDA for its Biosimilar Version of Xolair, CT-P39
Read More: Celltrion

Roche Reports Introduction of navify Digital Solutions for its Use in Laboratories and Point of Care Services at HIMSS 2024
Read More: Roche

Novartis to Acquire IFM Due (Subsidiary of IFM Therapeutics) for the Development of STING Antagonist Program
Read More: Novartis & IFM Therapeutics
For an Aggregate of ~$1.05B, AstraZeneca to Acquire Amolyt Pharma
Read More: AstraZeneca & Amolyt Pharma

The US FDA Grants Breakthrough Device Designation to SetPoint Medical’s Neuroimmune Modulation Platform for the Treatment of Multiple Sclerosis
Read More: SetPoint Medical
HeartBeam Reports Patient Enrollment in the Trial Investigating AIMIGo System for Arrhythmia Detection
Read More: HeartBeam

Cresilon Introduces Animal Philanthropy Program, VETIGEL Gives, to Provide Surgeries for Animals in Need
Read More: Cresilon
Related Post: PharmaShots Weekly Snapshots (March 04 – March 08, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














