PharmaShots Weekly Snapshots (February 19 – February 23, 2024)
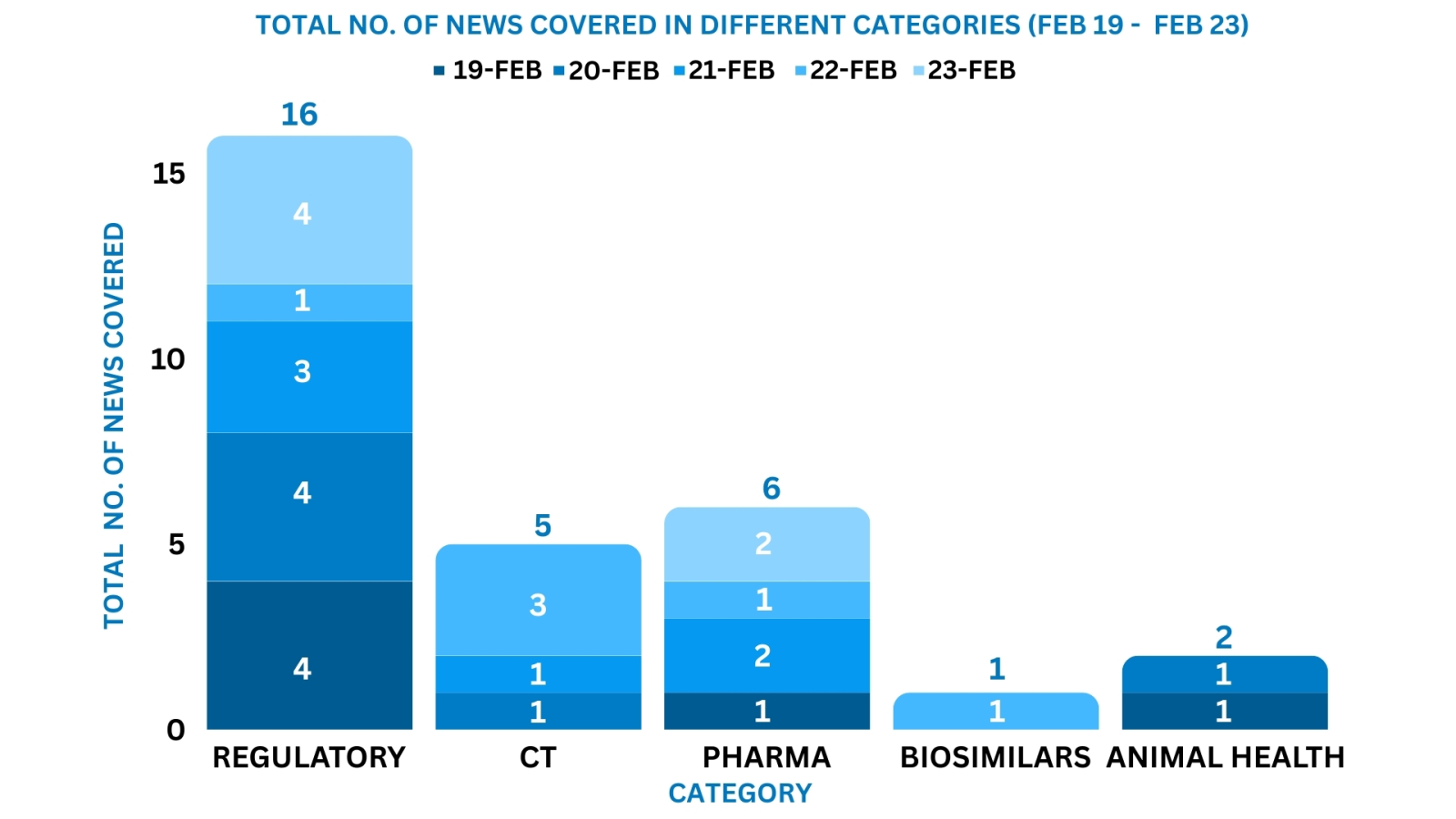
This week PharmaShots’ news was all about the updates on M&A, Pharma, Clinical Trials, Regulatory & MedTech. Check out our full report below:

Bavarian Nordic’s CHIKV VLP Receives EMA’s Accelerated Assessment for Chikungunya
Read More: Bavarian Nordic
The US FDA Approves Roche’s Xolair for Food Allergies
Read More: Roche
The US FDA Approves AstraZeneca’s Tagrisso, in Combination with Chemotherapy, for Treating EGFRm Advanced NSCLC
Read More: AstraZeneca
Iovance Receives the US FDA’s Accelerated Approval for Amtagvi (lifileucel) as a Treatment for Metastatic Melanoma (MM)
Read More: Iovance
The US FDA’s Accepts Daiichi Sankyo and AstraZeneca’s BLA for Datopotamab Deruxtecan to Treat Non-Small Cell Lung Cancer
Read More: Daiichi Sankyo & AstraZeneca
The European Commission Approves Pfizer’s Velsipity (etrasimod) for the Treatment of Severely Active Ulcerative Colitis
Read More: Pfizer
The US FDA Accepts and Grants Priority Review to argenx’s sBLA of Vyvgart Hytrulo for Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Read More: argenx
The US FDA Grants FTD to Certa Therapeutics’ FT011 for the Treatment of Systemic Sclerosis
Read More: Certa Therapeutics
The US FDA Accepts and Grants Priority Review to Merck’s sBLA for Keytruda Plus Chemotherapy to Treat Endometrial Carcinoma
Read More: Merck
The US FDA Accepts and Grants Priority Review to BMS’ sNDA for Krazati & Cetuximab Combination to Treat Colorectal Cancer (CRC)
Read More: BMS
RAPT Therapeutics Reports the US FDA’s Clinical Hold on the Studies Investigating Zelnecirnon
Read More: RAPT Therapeutics
The US FDA Approves Mabwell's IND Application for 7MW3711 as a Treatment for Solid Tumors
Read More: Mabwell
The US FDA Approves Johnson & Johnson’s sBLA of Tecvayli for Treating Relapsed/Refractory Multiple Myeloma
Read More: Johnson & Johnson
The US FDA Accepts and Grants Priority Review to Sanofi’s sBLA of Dupixent for the Treatment of COPD with Type 2 Inflammation
Read More: Sanofi
The US FDA Grants FTD to Artiva Biotherapeutics’ AlloNK (AB-101) for the Treatment of Lupus Nephritis
Read More: Artiva Biotherapeutics
The CHMP Grants Positive Opinion to CSL Vifor and Travere Therapeutics’ Sparsentan for the Treatment of IgA Nephropathy
Read More: CSL Vifor

Almirall and Novo Nordisk Collaborate to Develop NN-8828 for Treating Dermatological Diseases
Read More: Almirall & Novo Nordisk
Immune-Onc Therapeutics and Roche Partner to Assess IO-108 for the Treatment of Advanced Hepatocellular Carcinoma
Read More: Immune-Onc Therapeutics and Roche
Biocytogen and Gilead have Signed a Multi-Target Antibody Collaboration Agreement to Select and Develop Therapeutic Targets for the Treatment of Multiple Disease
Read More: Biocytogen & Gilead
Mainz Biomed Collaborates with TomaLab for Enhancing the Diagnosis of Colorectal Cancer Using ColoAlert
Read More: Mainz Biomed & TomaLab
Immunocore and BMS Collaborate for the Development of IMC-F106C in the P-III Trial for the Treatment of Melanoma
Read More: Immunocore & BMS
AbbVie and Tentarix Biotherapeutics Collaborate to Develop Biologics for Oncology and Immunology
Read More: AbbVie & Tentarix

Boehringer Ingelheim Collaborates with Sleip AI to Utilize AI-technology for Detecting Lameness in Horse
Read More: Boehringer Ingelheim & Sleip AI
Ono Pharmaceutical and InveniAI Sign a Research Collaboration Agreement for the Identification of Novel Therapeutic Targets Using AI and ML
Read More: Ono Pharmaceutical & InveniAI

Innovent Highlights the P-III (RESTORE-1) Trial Results of IBI311 to Treat Thyroid Eye Disease (TED)
Read More: Innovent
ViiV Healthcare (GSK & Pfizer’s Global Specialist HIV Company) Highlights P-III (LATITUDE) Trial Results of Cabenuva (cabotegravir + rilpivirine) for HIV
Read More: ViiV Healthcare
Aleta Biotherapeutics Doses First Patient with ALETA-001 in P-I/II Trial for the Treatment of R/R B-Cell Malignancies
Read More: Aleta Biotherapeutics
CymaBay Publishes P-III Results for Seladelpar as a Treatment of Primary Biliary Cholangitis in the New England Journal of Medicine
Read More: CymaBay
SynAct Pharma Highlights Further Analysis from the P-IIb (EXPAND) Study Supporting the Development of Resomelagonin for Rheumatoid Arthritis
Read More: SynAct Pharma

Mabwell Publishes the P-III Study Results of MW032 (Biosimilar, Denosumab) for Solid Tumors in JAMA Oncology
Read More: Mabwell
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.