
PharmaShots Weekly Snapshots (February 12 – February 16, 2024)
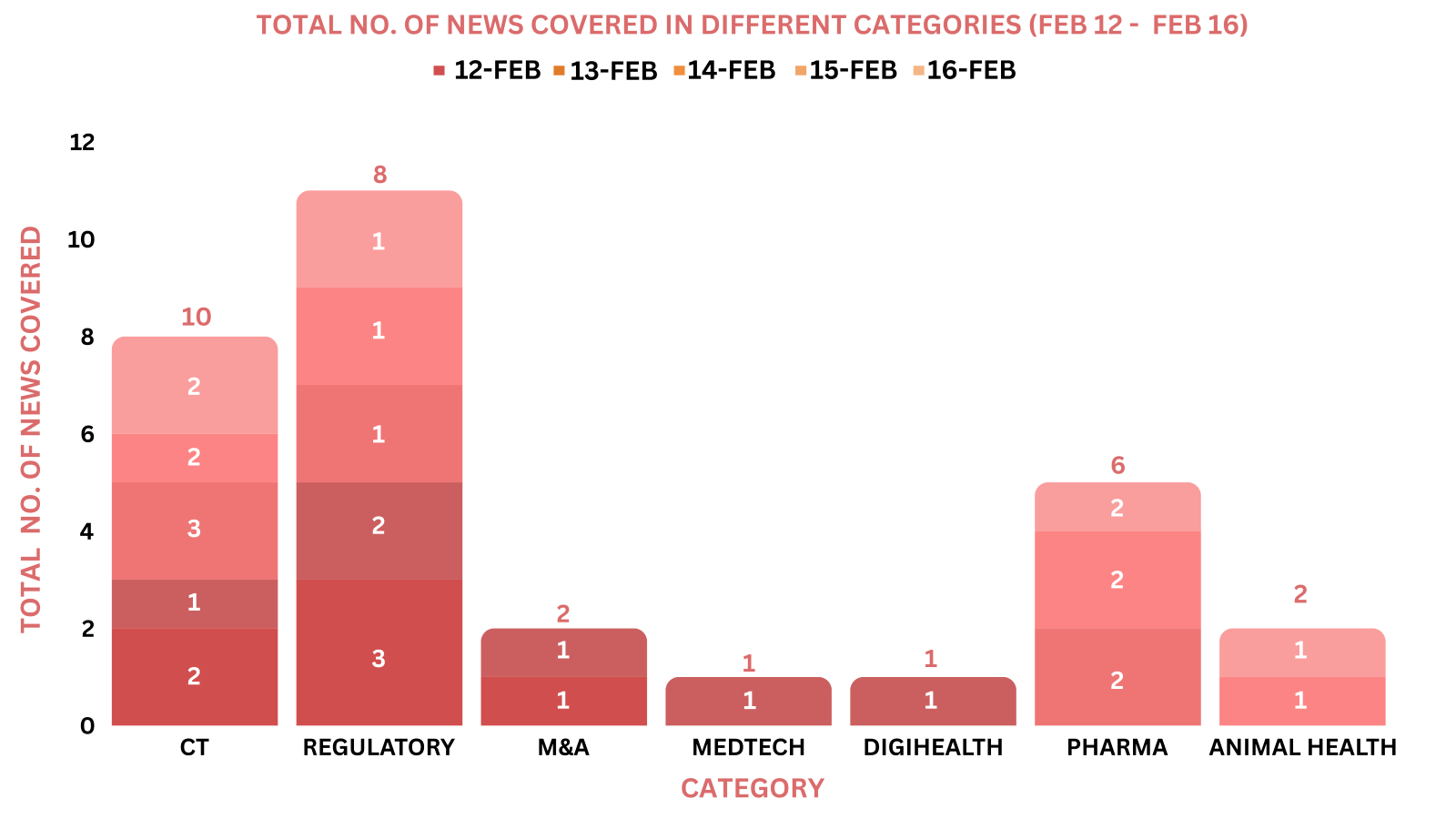
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, M&A, MedTech, DigiHealth, Pharma & Animal Health. Check out our full report below:

CSL Highlights Results from the P-III (AEGIS-II) Study of CSL112 for Acute Myocardial Infarction
Read More: CSL
Shionogi Reports the Results for Ensitrelvir in P-III Trial for the Treatment of Common COVID-19 Symptoms
Read More: Shionogi
Larimar Therapeutics Highlights the P-II Results for Nomlabofusp as a Treatment for Friedreich’s Ataxia
Read More: Larimar Therapeutics
KalVista Pharmaceuticals Reports the P-III (KONFIDENT) Study Results of Sebetralstat for Treating Hereditary Angioedema
Read More: KalVista Pharmaceuticals
Otsuka Highlights P-III Study Results of AVP-786 for Treating Agitation in Patients with Dementia due to Alzheimer's Disease
Read More: Otsuka
Replicate Bioscience Reports P-I Trial Results of RBI-4000 for Rabies
Read More: Replicate Bioscience
Sanofi Reports P-II Results for Frexalimab as a Treatment for Relapsing Multiple Sclerosis (MS)
Read More: Sanofi
Applied Therapeutics Highlights the P-III Results for Govorestat (AT-007) to Treat Sorbitol Dehydrogenase (SORD) Deficiency
Read More: Applied Therapeutics

The US FDA Accepts Humacyte’s and BLA for Human Aacelluar Vessel and Grants Priority Review for the Treatment of Vascular Trauma
Read More: Humacyte
The US FDA Granted the Fast Track Designation to GSK’s Bepirovirsen for the Treatment of Hepatitis B
Read More: GSK
The US FDA Grants BTD to AlphaMedix Developed Jointly by RadioMedix and Orano Med for Treating GEP-NETs
Read More: RadioMedix & Orano Med
Biogen’s Skyclarys (omaveloxolone) Receives the European Commission’s Approval for the Treatment of Friedreich’s Ataxia (FA)
Read More: Biogen
Takeda’s Eohilia (budesonide oral suspension) Received the US FDA’s Approval for the Treatment of Eosinophilic Esophagitis (EoE)
Read More: Takeda
The US FDA and the EMA Accepts Galderma’s BLA with Priority Review for Nemolizumab to Treat Nodularis and Atopic Dermatitis
Read More: Galderma
Ascentage Pharma Receives Clearance from the US FDA to Begin P-III Study of Olverembatinib for Chronic-Phase Chronic Myeloid Leukemia
Read More: Ascentage Pharma
Diamyd Medical’s Diamyd Receives US FDA’s Fast Track Designation for Type 1 Diabetes
Read More: Diamyd Medical
The US FDA Accepts and Grants Priority Review to BMS’ sNDA for Augtyro to Treat Solid Tumors
Read More: BMS
Merit Medical Receives the US FDA’s 510(k) Clearance for SCOUT MD Surgical Guidance System to Detect and Treat Soft Tissue Cancers
Read More: Merit Medical
The MHLW Granted Priority Review to Astellas’ sNDA for Padcev and Keytruda as Combination Therapy to Treat Bladder Cancer
Read More: Astellas

For an Aggregate of ~$0.81M, RaySearch Acquires Pharmacolog’s DrugLog
Read More: RaySearch & Pharmacolog
For an Aggregate of ~$4.3B, Gilead Sciences to Acquire CymaBay Therapeutics
Read More: Gilead Sciences & CymaBay Therapeutics

Tyber Medical’s Proximal Tibia Plating System Receives the US FDA’s 510(k) Clearance
Read More: Tyber Medical

Roche Signs an Agreement with PathAI to Enhance Digital Pathology Capabilities for Companion Diagnostics
Read More: Roche & PathAI

Ono Pharmaceutical and Shattuck Labs Partner to Develop Bifunctional Fusion Proteins for Treating Autoimmune and Inflammatory Diseases
Read More: Ono Pharmaceutical & Shattuck Labs
VantAI Joins Forces with BMS to Expedite Molecular Glue Drug Discovery Through Artificial Intelligence
Read More: VantAI & BMS
Erasca Signs two Clinical Trial Collaboration and Supply Agreements with Novartis to Develop Naporafenib Combinations for Treating Solid Tumors
Read More: Erasca & Novartis
Numab Therapeutics and Ono Pharmaceutical Collaborate to Develop Multi-specific Antibody, NM49, for Treating Cancer
Read More: Numab Therapeutics & Ono Pharmaceutical
Intellia Therapeutics Signs a Collaboration Agreement ReCode Therapeutics to Develop Novel Gene Editing Therapies for Cystic Fibrosis (CF)
Read More: Intellia Therapeutics & ReCode Therapeutics

Boehringer Ingelheim and Veeva System Partner to Simplify Clinical and Regulatory Operations in Animal Health
Read More: Boehringer Ingelheim & Veeva System
Jaguar Health Receives the US FDA Approval on the Clinical Trial Protocol for Canalevia-CA1 to Treat Chemotherapy-Induced Diarrhea in Dogs
Read More: Jaguar Health
Tags

Bharat is a data whiz who collate and analyzes the data to generate valuable insights, contributing to both our monthly reports and the extraction of key information from press releases. Through his work, Bharat plays a crucial role in ensuring Pharmashots delivers the most relevant and up-to-date information to our readers.














