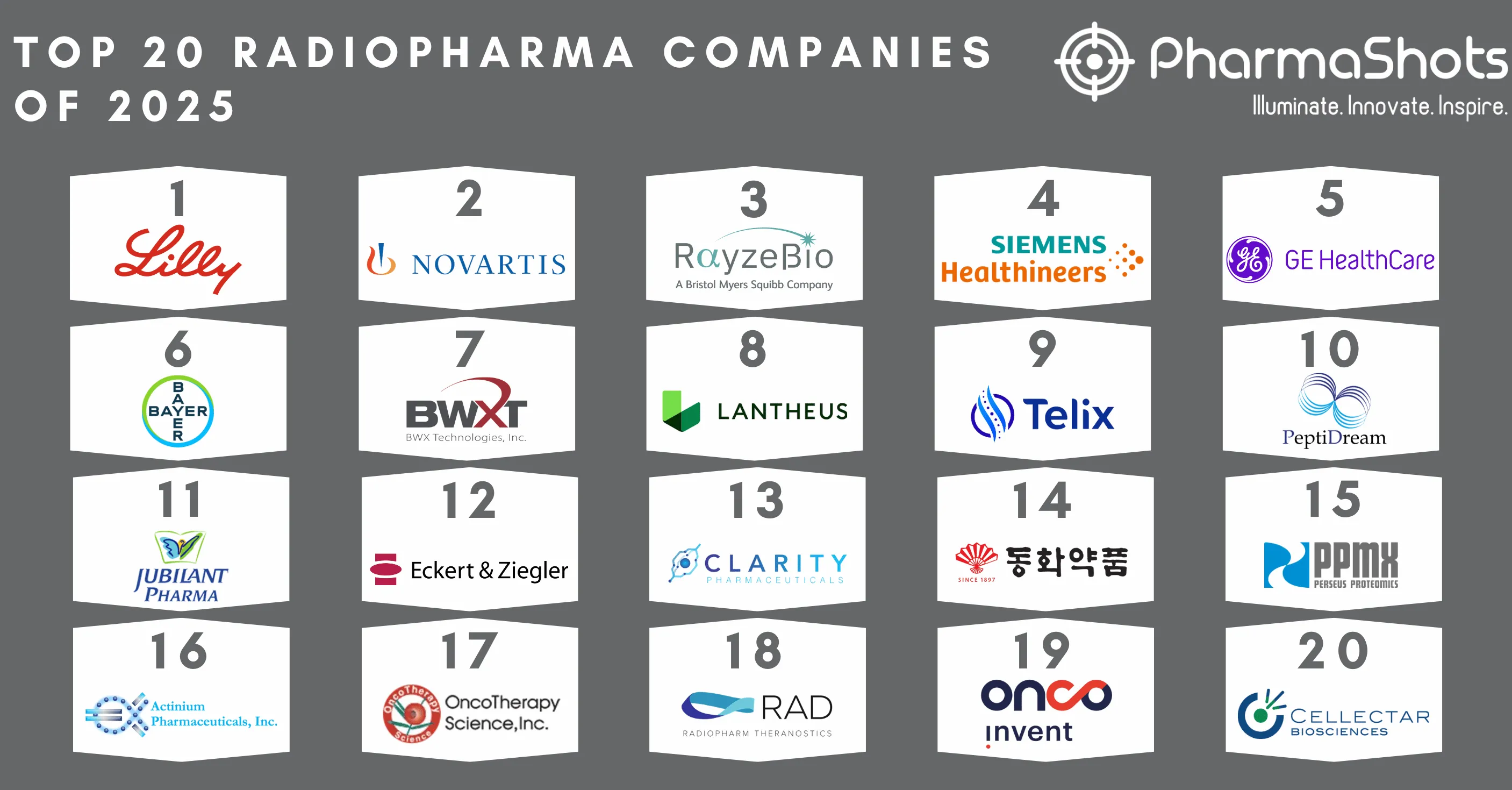
Top 20 Cell and Gene Therapy Companies Based on 2022 Total Revenue
Shots:
- Standing tall and gradually paving its way through extensive research, Cell & Gene Therapy is a beacon of hope when treating rare and inherited diseases. Cell and gene therapy aims to prevent, treat, and potentially reduce the effects of the underlying cause of genetic diseases.
- Propelled by constant innovatory winds, the cell and gene therapy market is anticipated to grow by 20 percent year-over-year through 2025. With a total revenue of $52B, Merck & Co. Was ranked the highest among the list of companies developing Cell & Gene therapy, followed by Novartis with a revenue of $50.54B and Spark Therapeutics with a revenue of $49.27B
- PharmaShots brings an informative report on the Top 20 Cell and Gene Therapy Companies based on the total Revenue generated in the year 2022

20. AnGes
Total Revenue: $0.5M
Approved Cell and Gene Therapy: Collategene
Founded Year: 1999
Total Employees: ~140
Headquarters: Osaka, Japan
Market Cap: $162.8M
Stock Exchange: TYO
With focus areas extending to the discovery and development of biopharma products, AnGes is dedicated to developing genetic medicines and therapeutic vaccines for intractable or rare diseases. The company received its first approval from the Japanese government in 2019 to treat critical limb ischemia. AnGes and Mitsubishi Tanabe Pharma jointly developed Collategene. This gene therapy delivers a plasmid expressing hepatocyte growth factor (HGF) into the lower limb muscles causing an improved local blood flow and ulcer healing.

19. Bluebird bio
Total Revenue: $3.6M
Approved Cell and Gene Therapy: Skysona & Zynteglo
Founded Year: 1992
Total Employees: ~323
Headquarters: Massachusetts, United States
Market Cap: $397.9M
Stock Exchange: NASDAQ
Committed to pharmaceutical products' research, development, and commercialization, bluebird bio utilizes its lentiviral vector gene addition platform to develop gene therapies for treating severe genetic diseases. The company first received the US FDA approval for its gene therapy Zynteglo in Aug 2022 for treating adult and pediatric patients with ß-thalassemia. Zynteglo adds functional copies of the modified β-globin gene to the patient's hematopoietic stem cells (HSCs). Later in Sep 2022, the company also received approval from the US FDA for its gene therapy, Skysona, to treat boys aged between 4 and 17 with active cerebral adrenoleukodystrophy (CALD). Skysona functions by lowering the progression of neurologic dysfunction in CALD patients. In November 2021, Bluebird Bio spun off its Oncology business into a separate company named 2seventy Bio.

18. Orchard Therapeutics
Total Revenue: $20M
Approved Cell and Gene Therapy: Libmeldy & Strimvelis
Founded Year: 2015
Total Employees: ~170
Headquarters: London, United Kingdom
Market Cap: $100.6M
Stock Exchange: NASDAQ
Orchard Therapeutics is a company that develops and commercializes gene therapies to treat the underlying cause of diseases. The company has two approved gene therapies, Libmeldy and Strimvelis. Libmeldy received marketing authorization from the EMA on Dec 2020 for the treatment of Metachromatic Leukodystrophy (MLD), whereas, Strimvelis obtained the EMA’s approval in May 2016 for the treatment of Adenosine Deaminase Deficiency. Libmeldy is a genetically modified autologous CD34+ cell-enriched population containing HSPC transduced ex vivo using a lentiviral vector expressing the human arylsulfatase A (ARSA) gene. Strimvelis, an autologous CD34+ enriched cell fraction, is composed of CD34+ cells that have been retrovirally transduced with a retroviral vector that encodes for the human adenosine deaminase (ADA) cDNA sequence from human hematopoietic stem/progenitor (CD34+) cells.

17. JW Therapeutics
Total Revenue: $21M
Approved Cell and Gene Therapy: Carteyva (Relma-cel)
Founded Year: 2016
Total Employees: ~90
Headquarters: Shanghai, China
Market Cap: $165.05M
Stock Exchange: HKG
Marking its position in the Cell and Gene Therapy market, JW Therapeutics is a biotech company working on developing, manufacturing, and commercializing cell immunotherapy products. The company develops therapies for therapeutic areas, including hematologic malignancies, solid tumors, and autoimmune diseases. Its Carteyva (Relma-cel) received the NMPA's approval in Sep 2021 for treating large B-cell lymphoma. Carteyva is an autologous anti-CD19 CAR-T cell immunotherapy developed by JW Therapeutics using Juno Therapeutics' CAR-T cell process platform.

16. Organogenesis
Total Revenue: $45M
Approved Cell and Gene Therapy: Apligraf & Novachor
Founded Year: 1985
Total Employees: ~1,030
Headquarters: Massachusetts, United States
Market Cap: $420.04M
Stock Exchange: NASDAQ
A regenerative medicine company, Organogenesis is a pharma company that develops, manufactures, and commercializes Advanced Wound Care and Surgical and Sports Medicines. Apligraf and Novachor are the two approved gene therapies developed by the company. Apligraf, approved in 1998, is a bioengineered living cell therapy containing keratinocytes and fibroblasts producing a wide range of cytokines and growth factors. Approved in the year 2021, Novachor is a fresh chorion membrane wound covering developed as a preservative of viable cells, growth factors, cytokines, and ECM proteins in native tissue.

15. 2seventy bio
Total Revenue: $91.49
Approved Cell and Gene Therapy: Abecma
Founded Year: 2021
Total Employees: ~425
Headquarters: Massachusetts, United States
Market Cap: $0.40B
Stock Exchange: NASDAQ
2seventy bio develops and commercializes cell and gene therapy in cancer. Through research and development, the Abecma (idecabtagene vicleucel, or ide-cel) became the first FDA-approved CAR T cell therapy for multiple myeloma. The company has DARIC33 and bbT369 in its pipeline. Abecma is a CAR T cell treatment that detects and attaches to BCMA on the surface of multiple myeloma cells, causing CAR T cell proliferation, cytokine production, and cytolytic death of BCMA-expressing cells. Abecma generated total revenue of $12.78M and contributed to the company’s total revenue in collaboration with BMS. Abecma has been developed and commercialized in the US as part of a co-development, co-promotion, and profit-sharing agreement between BMS & 2seventy Bio.

14. uniQure
Total Revenue: $110M
Approved Cell and Gene Therapy: Hemgenix
Founded Year: 1998
Total Employees: ~500
Headquarters: Amsterdam, Netherlands
Market Cap: $1.01B
Stock Exchange: NASDAQ
Delving into the Cell and Gene Therapies market, uniQure is a biopharma company that develops and commercializes gene therapies to treat rare diseases. Amongst the pipeline of gene therapy being evaluated, In Nov 2022, the company received approval for Hemgenix from the US FDA to treat Hemophilia B. Hemgenix is an adeno-associated virus vector serotype 5 (AAV5) based gene therapy designed to deliver a copy of a gene encoding Padua variant of human coagulation Factor IX (hFIX-Padua)

13. Legend Biotech (Jannsen)
Total Revenue: $117M
Approved Cell and Gene Therapy: Carvykti
Founded Year: 2014
Total Employees: ~1,400
Headquarters: New Jersey, United States
Market Cap: $11.89B
Stock Exchange: NASDAQ
Legend Biotech is a multinational biotech company that develops, manufactures, and commercializes therapies for treating life-threatening diseases. It is a holding company that conducts operations through its subsidiaries, including its PCR subsidiaries. In Dec 2017, Legend Biotech entered into a collaboration agreement with Janssen Pharmaceutical to develop and commercialize Carvykti. The company’s gene therapy candidate, Carvykti, received the US FDA's approval in Mar 2022 for treating Multiple Myeloma, according to the NDA filed by Janssen. It also received approval from the EMA and MHLW in May and Sep 2022. Carvykti is a BCMA-directed genetically modified autologous T-cell immunotherapy that involves the reprogramming of the patient's T-cells with a transgene encoding a CAR that identifies and eliminates BCMA-expressing cells

12. Vericel Corp.
Total Revenue: $164M
Approved Cell and Gene Therapy: MACI
Founded Year: 1987
Total Employees: ~300
Headquarters: Massachusetts, United States
Market Cap: $1.55B
Stock Exchange: NASDAQ
A biopharma company marking its way into the Cell and Gene Therapies Market, Vericel Corp. develops and commercializes gene therapies targeting unmet therapeutic needs. MACI is the company’s only approved product to date. The company received the US FDA’s approval for MACI in Dec 2016 for the repair therapy of cartilage defects in patients with knee problems. MACI is developed using the patient’s autologous cells expanded and placed into a bio-resorbable porcine-derived collagen membrane implanted over the area from which the defective or damaged tissue was removed.

11. JCR Pharmaceuticals
Total Revenue: $302M
Approved Cell and Gene Therapy: Temcell
Founded Year: 1975
Total Employees: ~800
Headquarters: Ashiya, Japan
Market Cap: $1.30B
Stock Exchange: TYO
Aimed toward the research, development, manufacturing, and marketing of biopharma and regenerative products, JCR Pharmaceuticals has a well-established position in the Cell and Gene Therapies Market. The company utilizes technologies for cell therapy, regenerative medicines, and gene therapy technology to develop drugs and drug discovery platforms. JCR’s allogeneic cell therapy product, Temcell, was approved by the MHLW in Feb 2016 for the treatment of acute graft versus host disease (aGVHD) in children and adults.

10. PTC Therapeutics
Total Revenue: $530M
Approved Cell and Gene Therapy: Upstaza
Founded Year: 1998
Total Employees: ~1,400
Headquarters: New Jersey, United States
Market Cap: $4.33B
Stock Exchange: NASDAQ
PTC Therapeutics discovers, develops, and commercializes products, including gene therapies, for treating rare diseases. Upstaza is the company’s approved gene therapy. In Jul 2022, the EMA granted Upstaza marketing authorization to treat adults and children aged 18 years or above with severe aromatic L-amino acid decarboxylase (AADC). Upstaza contains a functional version of the AADC gene, eladocagene exuparvovec, within a modified virus.

09. Mallinckrodt
Total Revenue: $1.9B
Approved Cell and Gene Therapy: StrataGraft
Founded Year: 1867
Total Employees: ~2,700
Headquarters: Dublin, Ireland
Market Cap: $82.98M
Stock Exchange: NYSE
Mallinckrodt is a biopharma company focused on the development, manufacturing, marketing, and commercialization of pharma products and therapies for therapy areas, including Autoimmune and Rare Diseases, with a specialty in Neurology, Rheumatology, Hepatology, Nephrology, Pulmonology, Ophthalmology, and Oncology. The company's cell therapy portfolio contains a US FDA-approved StrataGraft. StrataGraft was approved by the US FDA in Jun 2021 for treating adults with deep partial-thickness burns. Keratinocytes and dermal fibroblasts are the two human skin cells incorporated in the development of StrataGraft, which are grown together to form a bi-layered construct on the skin.

08. BioMarin
Total Revenue: $2.09B
Approved Cell and Gene Therapy: Roctavian
Founded Year: 1997
Total Employees: ~3,100
Headquarters: California, United States
Market Cap: $17.25B
Stock Exchange: NASDAQ
BioMarin is a global biopharma company that develops and commercializes targeted therapies for genetic disorders. The company has Roctavian as its approved gene therapy. In Aug 2022, BioMarin received conditional marketing authorization for Roctavian from the EMA for treating severe hemophilia A. Roctavin is developed using valoctocogene roxaparvovec as the active gene. The product carries and delivers a short and functional copy of the F8 gene to the liver cells by using AAV5, an adeno-associated virus.

07. Daiichi Sankyo
Total Revenue: $10.53B
Approved Cell and Gene Therapy: Delytact
Founded Year: 2005
Total Employees: ~16,450
Headquarters: Tokyo, Japan
Market Cap: $68.27B
Stock Exchange: TYO
Daiichi Sankyo discovers and develops products for therapy areas, including Oncology and Rare Diseases. Delytact is the company's approved gene therapy. In Jun 2021, the MHLW approved Daiichi to manufacture and market Delytact across Japan to treat patients with malignant glioma. Delytact is a genetically engineered oncolytic herpes simplex virus type 1 (HSV-1). It has triple mutation within the viral genome that causes augmented and selective replication in cancer cells and enhances the induction of antitumor immune response.

06. Amgen
Total Revenue: $26.32B
Approved Cell and Gene Therapy: Imlygic
Founded Year: 1980
Total Employees: ~25,200
Headquarters: California, United States
Market Cap: $119.38B
Stock Exchange: NASDAQ
Amgen is an American biotech company that discovers, develops, and manufactures products targeting diseases with limited treatment options. The company has marked its spot in the cell and gene market through its approved gene therapy, Imlygic. Amgen received the US FDA’s approval for Imlygic in Oct 2015 and the EMA's approval in Dec 2015 for treating unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after the initial surgery. Imlygic is a genetically modified herpes simplex virus type 1, which, when injected inside the tumor, replicates and produces an immunostimulatory protein, GM-CSF.

05. Kite Pharma (Gilead)
Total Revenue: $27.28B
Approved Cell and Gene Therapy: Tecartus & Yescarta
Founded Year: 1987
Total Employees: ~17,000
Headquarters: California, United States
Market Cap: $98.35B
Stock Exchange: NASDAQ
An American Biotech company, Kite Pharma, develops immunotherapy products for the treatment of cancers. Kite became an integral part of Gilead Sciences following its acquisition in 2017 by Gilead Sciences. The company primarily focuses on developing genetically engineered CAR T cell therapies. Tecartus & Yescarta are two approved CAR-T cell therapies developed by Kite Pharma. Tecartus was approved by the EMA (Dec 2020) and US FDA (Oct 2021), whereas, Yescarta was approved by the EMA (Aug 2018), TGA (Feb 2020), MHLW (Jan 2021; filed by Daiichi Sankyo), US FDA (Apr 2022), and Health Canada (Mar 2023) for the treatment of Mantle cell lymphoma.

04. Takeda
Total Revenue: $29.40B
Approved Cell and Gene Therapy: Alofisel
Founded Year: 1781
Total Employees: ~50,000
Headquarters: Tokyo, Japan
Market Cap: $51.24B
Stock Exchange: TYO
An R&D-driven global biopharma company, Takeda is committed to discovering and delivering products across therapy areas, including Oncology, Metabolic Disorders, Gastroenterology (GI), Neurology, and Immunology. Takeda's cell and gene therapy segment includes Alofisel. The MHLW approved Takeda to manufacture and commercialize Alofisel across Japan to treat patients with non-active or mildly active Luminal Crohn's Disease. The suspension of allogeneic develops Alofisel expanded adipose-derived stem cells (eASC) to treat complex perianal fistulas in patients with Luminal Crohn's Disease.

03. Celgene (Bristol Myers Squibb)
Total Revenue: $46.16B
Approved Cell and Gene Therapy: Breyanzi & Abecma
Founded Year: 1887
Total Employees: ~34,300
Headquarters: New York, United States
Market Cap: $138.32B
Stock Exchange: NYSE
Bristol Myers Squibb (BMS) is a global biopharma company dedicated to developing and commercializing products targeting therapy areas, including Oncology, Cardiovascular, and Immunological Diseases. BMS's subsidiary, Celgene Corporation, is a pharmaceutical company primarily focusing on developing cancer treatment immunotherapies. Under its immunology cell therapy, Celgene has two approved therapies, Breyanzi and Abecma. Breyanzi was approved by the MHLW (Mar 2021), EMA (Apr 2022), and the US FDA (Jun 2022) for the treatment of large B-cell lymphoma. Breyanzi is a CAR T cell therapy that targets CD19 expressed on the Cell during normal B-cell development. Additionally, in collaboration with 2seventybio (a bluebird bio company), BMS also received approval for Abecma from the US FDA (Mar 2021), Health Canada (May 2021), and the EMA (Aug 2021) for the treatment of multiple myeloma. Abecma is a BCMA-directed CAR T immunotherapy.

02. Spark Therapeutics (Roche)
Total Revenue: $49.27B
Approved Cell and Gene Therapy: Luxturna
Founded Year: 1896
Total Employees: ~1,03,600
Headquarters: Basel, Switzerland
Market Cap: $308.07B
Stock Exchange: SWX
Roche is a multination biopharma company that develops and commercializes products under therapy areas, including Oncology, Cardiovascular, and Immunological Diseases. Spark Therapeutics' subsidiary discovers and delivers gene therapies for treating genetic disorders. Spark's Luxturna was approved by the US FDA (Dec 2017), EMA (Nov 2018), TGA (Aug 2020), and Health Canada (Oct 2020) as a gene therapy for the treatment of Leber's congenital amaurosis. Luxturna is developed using the active substance vortigene neparvovec. Earlier in Jan 2018, Spark Therapeutics entered into a licensing agreement with Novartis to develop and commercialize Luxturna in areas outside the US.

01. Novartis
Total Revenue: $50.54B
Approved Cell and Gene Therapy: Zolgensma & Kymriah
Founded Year: 1996
Total Employees: ~102,000
Headquarters: Basel, Switzerland
Market Cap: $229.11B
Stock Exchange: SWX
Novartis is a global biopharma company that discovers, develops, and delivers pharma products to treat unmet medical conditions in therapy areas, including Oncology, Hepatology, Immunology, Dermatology, Musculoskeletal Diseases, Neurosciences, and Ophthalmology. Kymriah is the company's first cell therapy approved for treating acute lymphocytic leukemia. Kymriah was approved by the EMA (Aug 2018), Health Canada (Sep 2018), TGA (Dec 2018), MHLW (Mar 2019), and the US FDA (May 2022). Kymriah is a T-cell therapy that utilizes the patient's T cells to fight and kill cancer cells. Zolgensma is Novartis' approved gene therapy for treating spinal muscular atrophy (SMA). It has received approval from the US FDA (May 2019), MHLW (Mar 2020), EMA (May 2020), and Health Canada (Dec 2020) for the treatment of SMA. Zolgensma replaced the missing or defective SMN1 gene with a new copy of the gene in the patient's Cell, thereby inhibiting disease progression.
Sources:
- Annual reports
- SEC Filings
- Press releases
- Company websites
Market Cap Source: Google Finance (20th May 2023)
Currency Conversion: X-Rates (20th May 2023)
Note:
- All market caps were market on 20th May 2023
Related Posts: Top 20 RNAi Therapeutic Companies Based on 2022 R&D Expenditure
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.