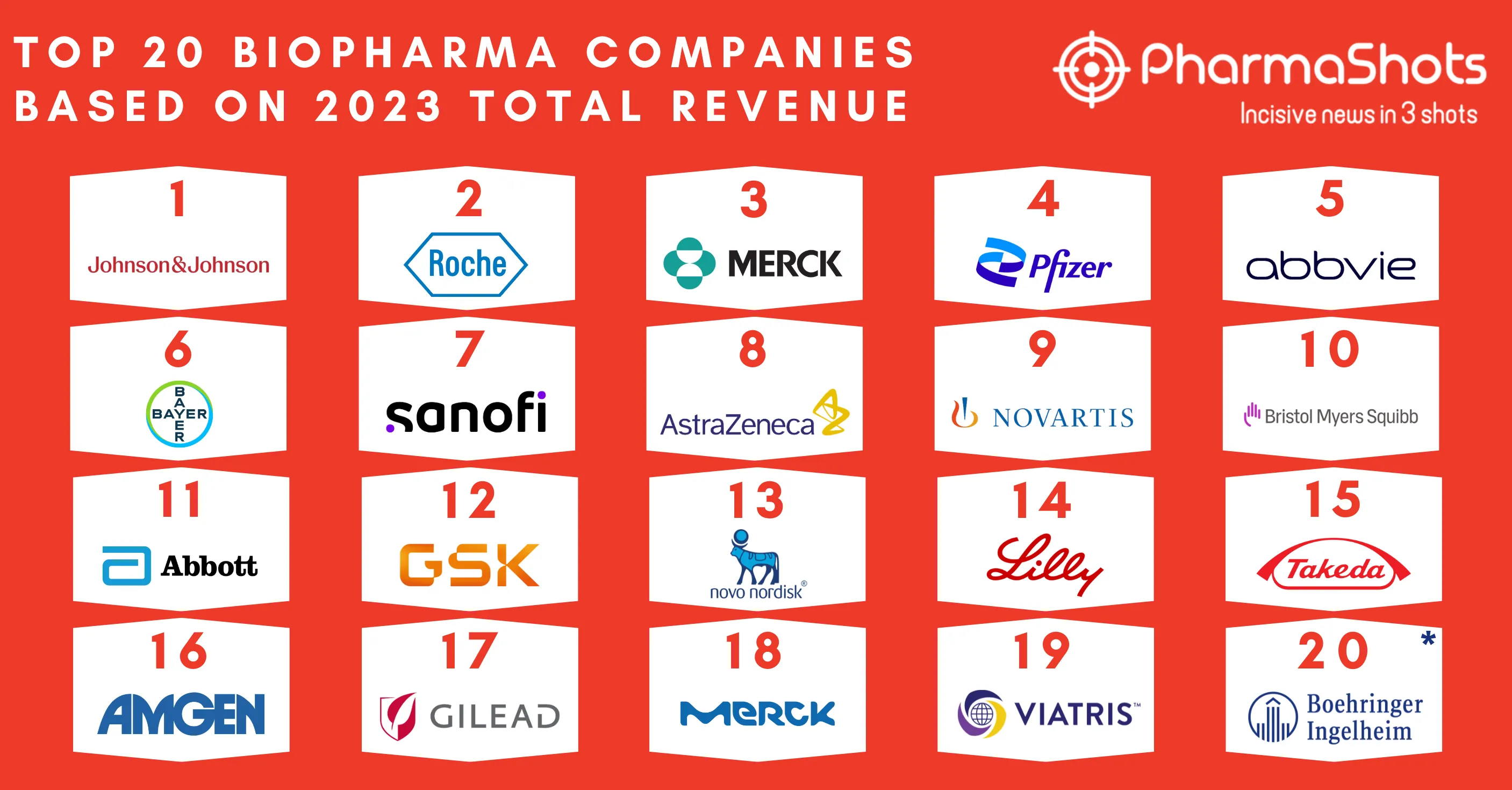
Linnaeus Therapeutics Reports the Expansion of Clinical Collaboration with Merck to Evaluate LNS8801 + Keytruda for the Treatment of Patients with Advanced Cancer
Shots:
- Linnaeus will conduct 6 additional P-II cohorts evaluating LNS8801 + Keytruda in several cancer indications while the companies are currently evaluating the combination in patients who had previously responded to PD-1/L1 therapy and also LNS8801 as a monothx in patients unable to tolerate PD-1/L1 therapy due to serious immune-related AEs
- The companies will further evaluate the combination in patients with selected advanced solid tumors- based on promising preliminary safety- PD- and efficacy data
- LNS8801 (PO) is a bioavailable small molecule that is a highly specific & potent agonist of the GPER and ongoing P-I/IIa study demonstrates target engagement- c-Myc oncoprotein depletion- and clinical beneficial for advanced cancer patients
Ref: PR Newswire | Image: Merck
Click here to read the full press release

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com