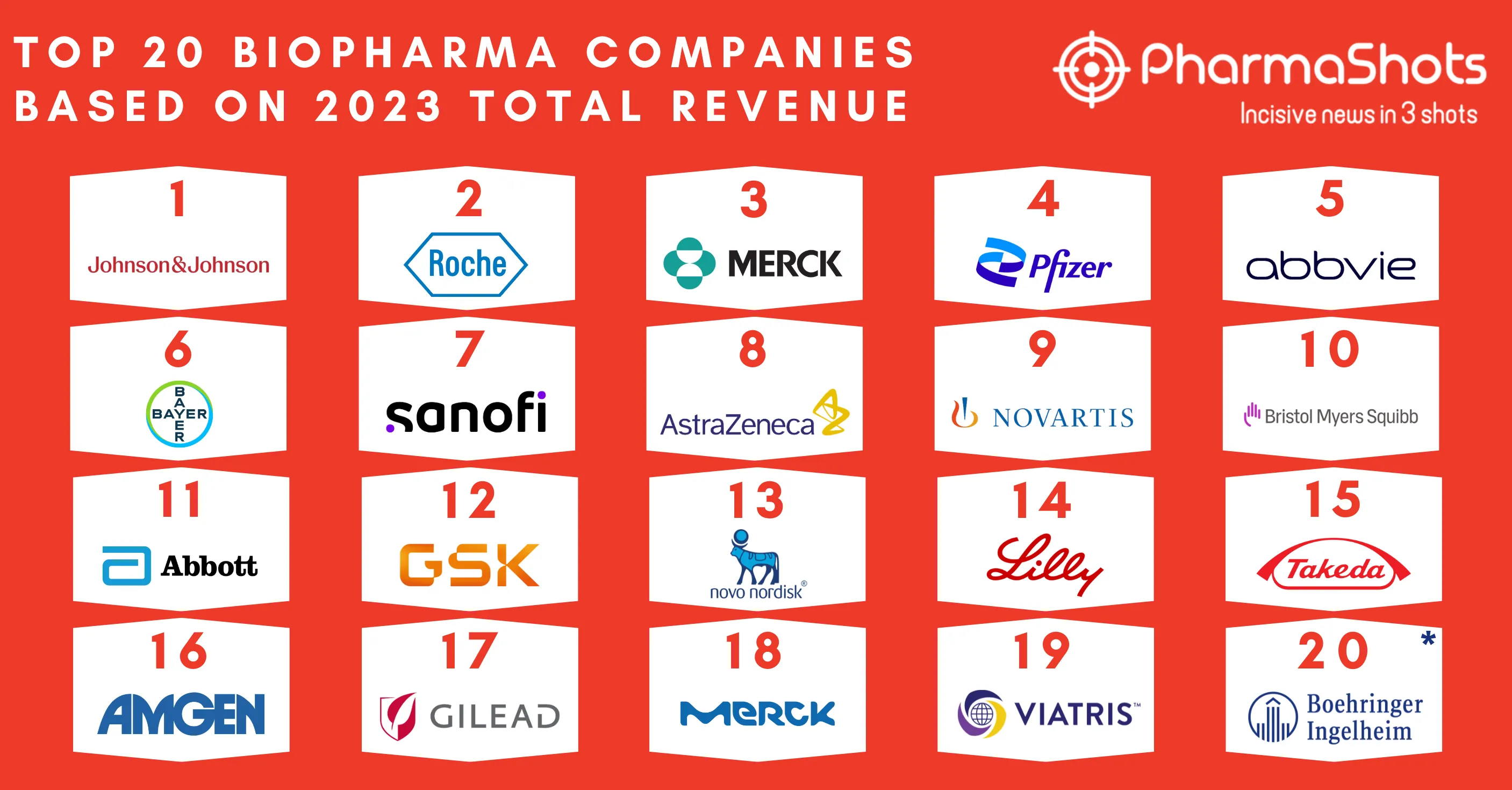
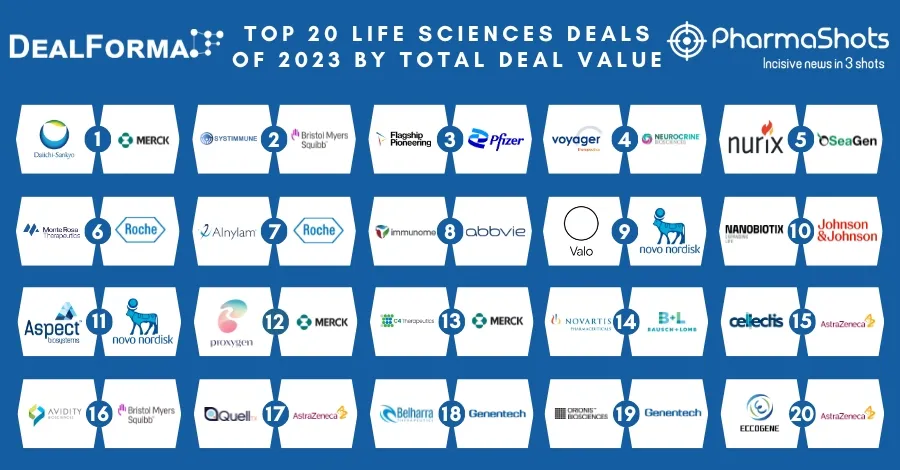
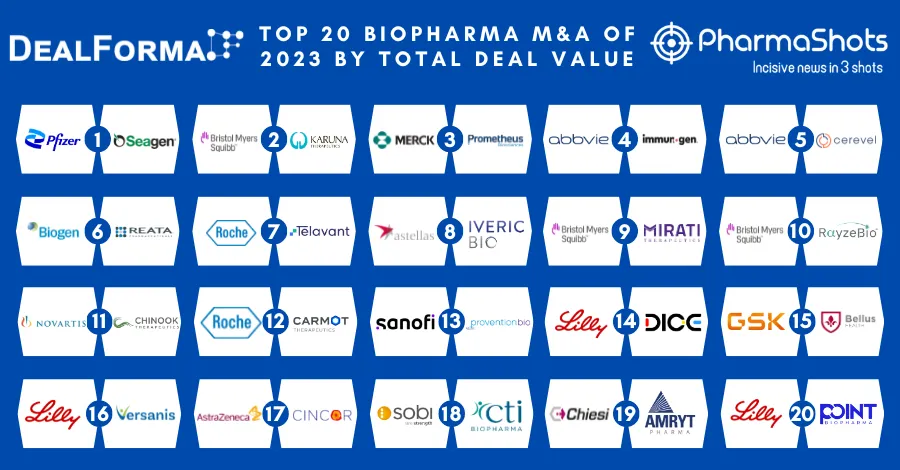
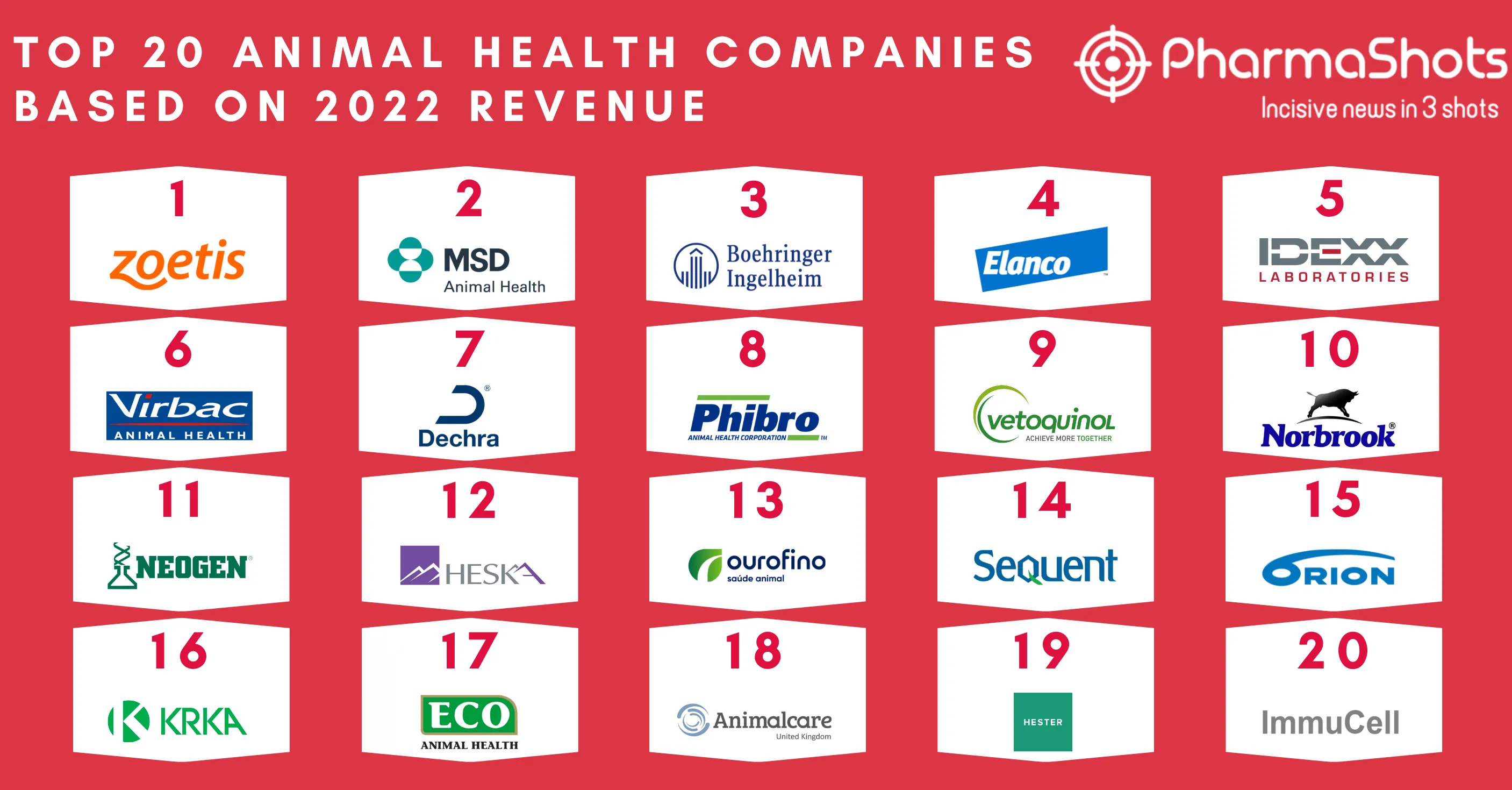
Eisai's and Merck Announces EU's Marketing Approval of Lenvima (lenvatinib mesylate) for Hepatocellular carcinoma
Shots:
- Post FDA approval- Lenvima receives EU approval for oral RTKi for the treatment of advanced or unresectable HCC
- The approval is based on REFLECT study results: (Lenvima vs Sorafenib): mOS (13.6 vs 12.3 mos)- mPFS (7.3 vs 3.6 mos); ORR (41% vs 12%); mTTP (7.4 vs 3.7mos)
- Lenvima is approved for thyroid cancer in over 50 countries including in EU- Japan and the US- and in EU it is available as Kisplyx in combination with everolimus for the 2L treatment of renal cell carcinoma
Ref: Eisai | Image: Merck
Click here to read the full press release
Tags

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com