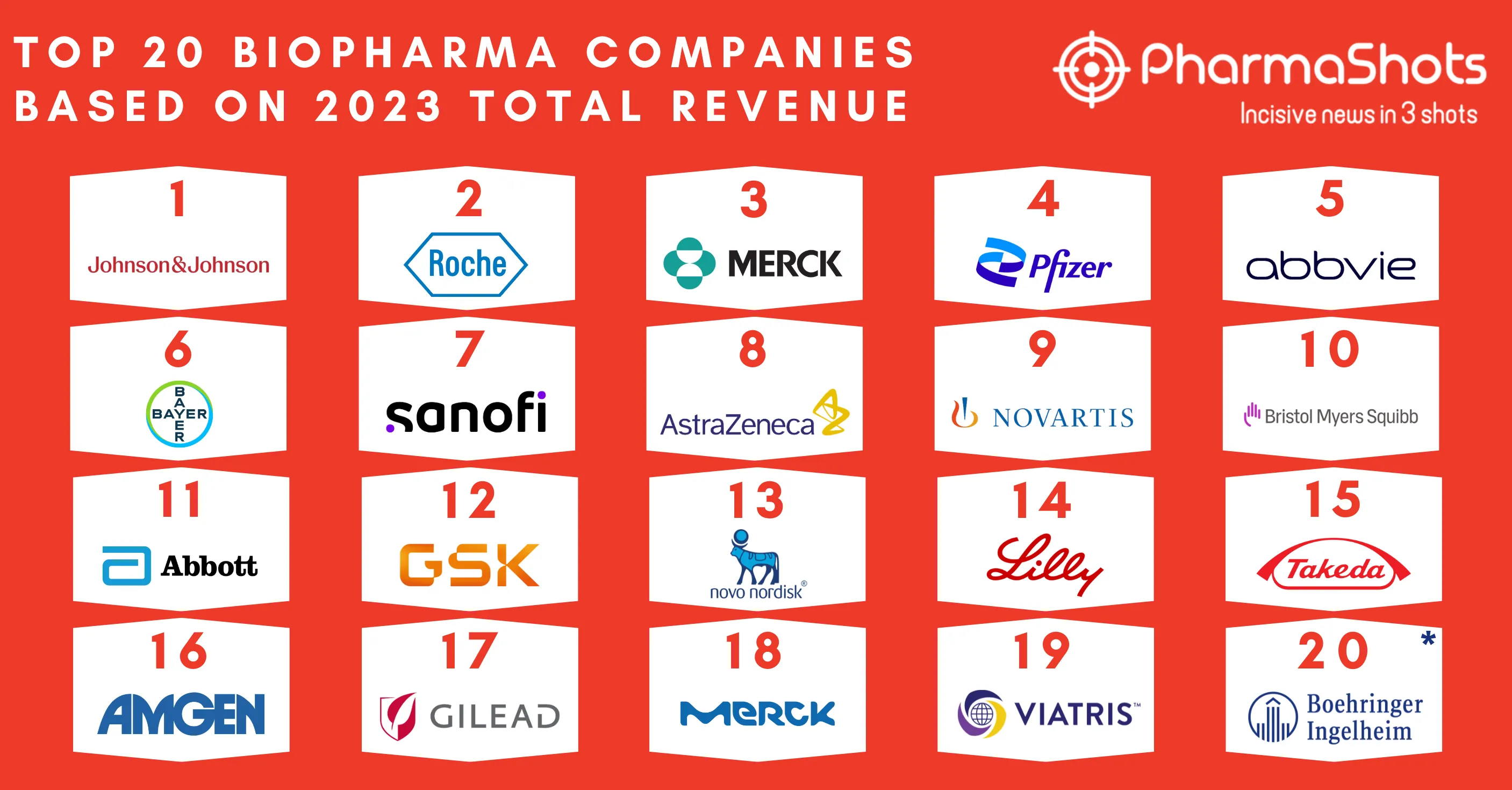
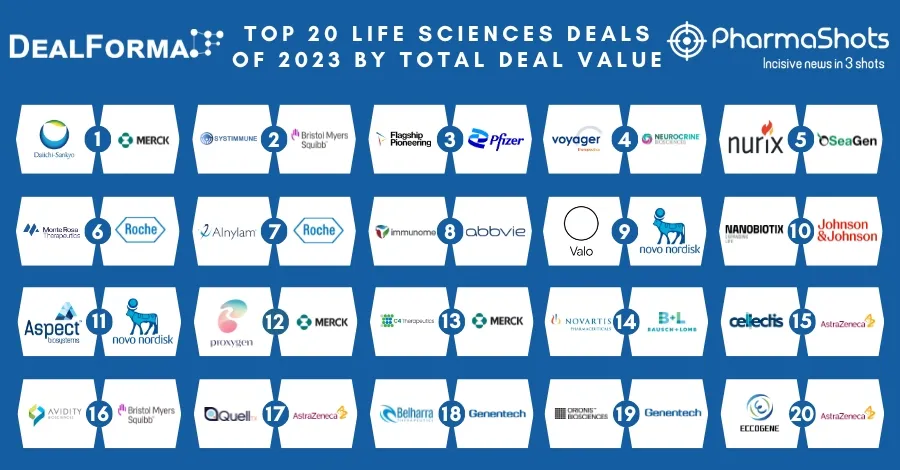
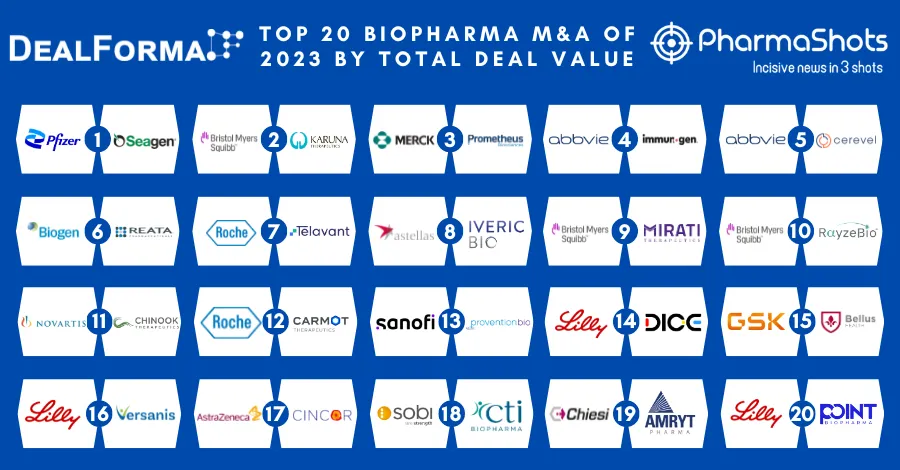
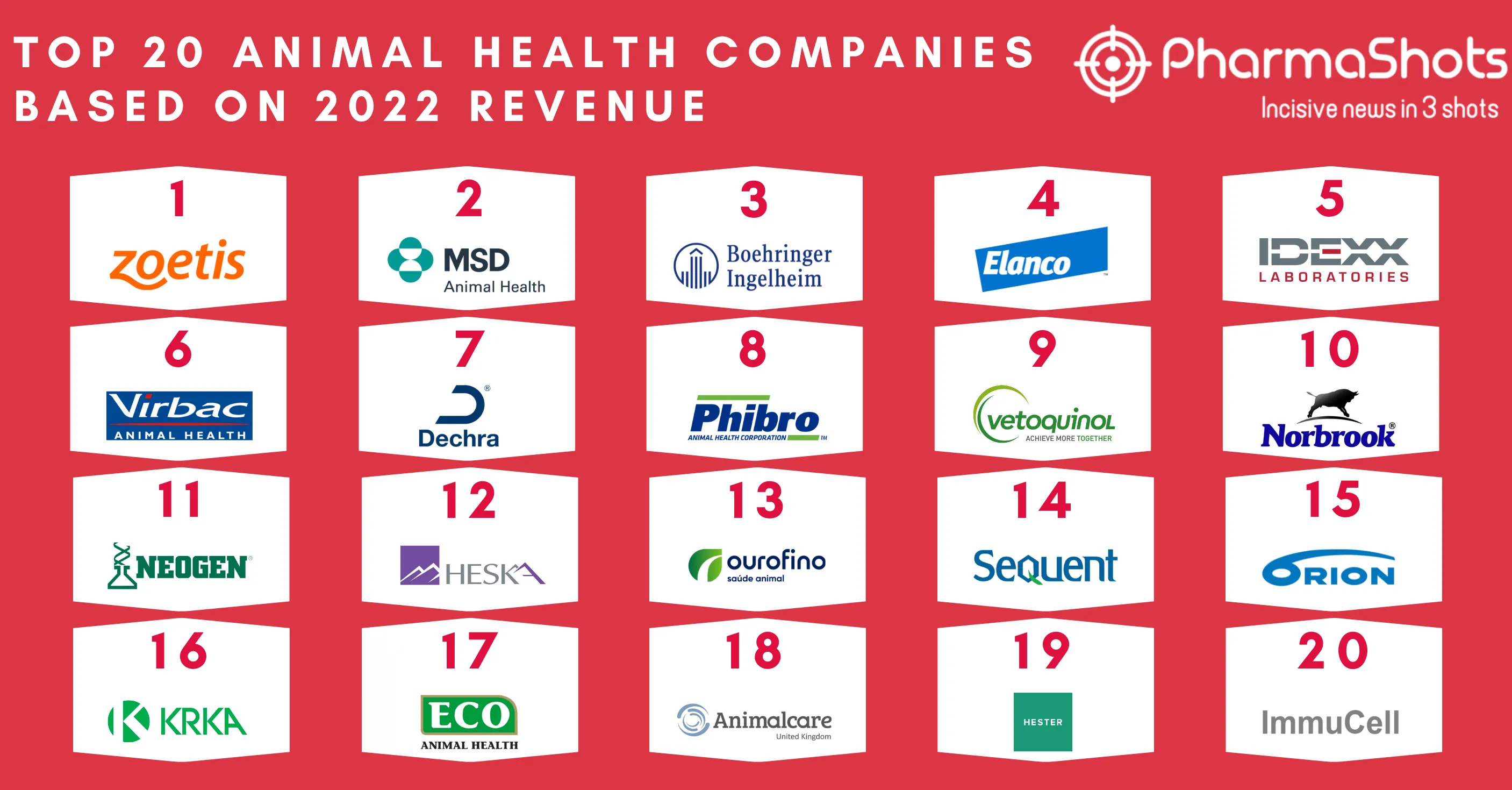
Gilead Collaborates with Merck to Jointly Develop and Commercialize Lenacapavir + Islatravir for HIV
Shots:
- The companies will co-develop & co-commercialize the combination of Gilead’s Lenacapavir and Merck’s Islatravir in long-acting oral & injectable formulations for HIV. Gilead & Merck will share global development & commercialization costs (60%/40%) respectively
- For oral products- Gilead will lead commercialization in the US & Merck will lead in the EU & ROW while for injectables- commercialization will be vice versa. Additionally- each company will exercise its option to acquire the license for an investigational oral integrase inhibitor of the other company- following completion of the first P-I trial
- Upon exercise of an option- the companies will split development cost & revenues- unless the non-exercising company decides to opt-out. Both companies’ integrase inhibitors are currently in preclinical development
Ref: Businesswire | Image: Gilead
Click here to read the full press release
Tags

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com