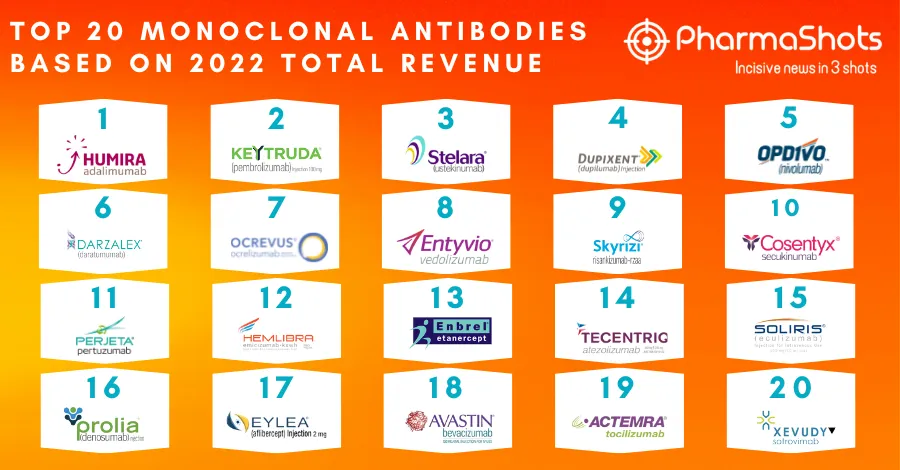
ViiV Healthcare's First Long-Acting Injectable Receive EC's Approval for the Treatment of HIV
Shots:
- The approval is based on P-III ATLAS- FLAIR and ATLAS-2M studies assessing Vocabria (cabotegravir injection and tablets) + Janssen’s Rekambys (rilpivirine inj.) or Edurant (rilpivirine tablets) in 1200+ patients for HIV-1 infection in adults who are virologically suppressed
- The first long-acting injectable can enable people living with HIV to reduce the days they receive treatment from 365 to 12 or 6/year. Most of the clinical trial patients who tried the treatment over their previous daily oral therapy preferred the new treatment
- The approval marks the second approval of the long-acting regimen of cabotegravir and rilpivirine- with once-monthly dosing licensed by Health Canada under the brand name Cabenuva
Ref: Businesswire | Image: The Pharma Letter
Click here to read the full press release

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com