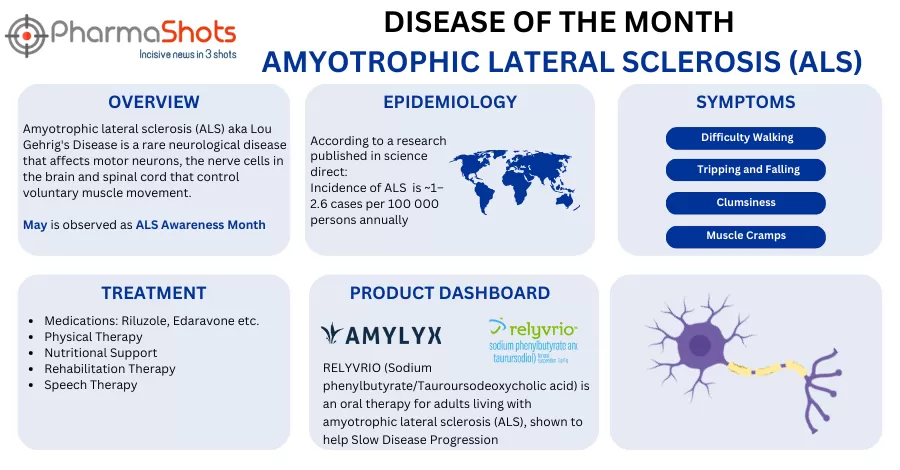
Project ALS' Prosetin Receives the US FDA's Orphan Drug Designation for Amyotrophic Lateral Sclerosis
Shots:
- The US FDA has granted ODD to Prosetin for the treatment of ALS. ODD provides incentives for the development of drugs- biologics- and devices for diseases affecting 200-000 or fewer Americans
- By obtaining ODD- Prosetin is now eligible for benefits including up to 7yrs. of marketing exclusivity if it receives regulatory approval- exemption from FDA user fees- FDA assistance in clinical trial design- and tax credits for clinical research
- Prosetin is an oral- brain penetrant MAP4 kinase inhibitor- developed at Columbia University for the treatment of ALS
Ref: PRNewswire | Image: Twitter
Click here to read the full press release
Tags

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com