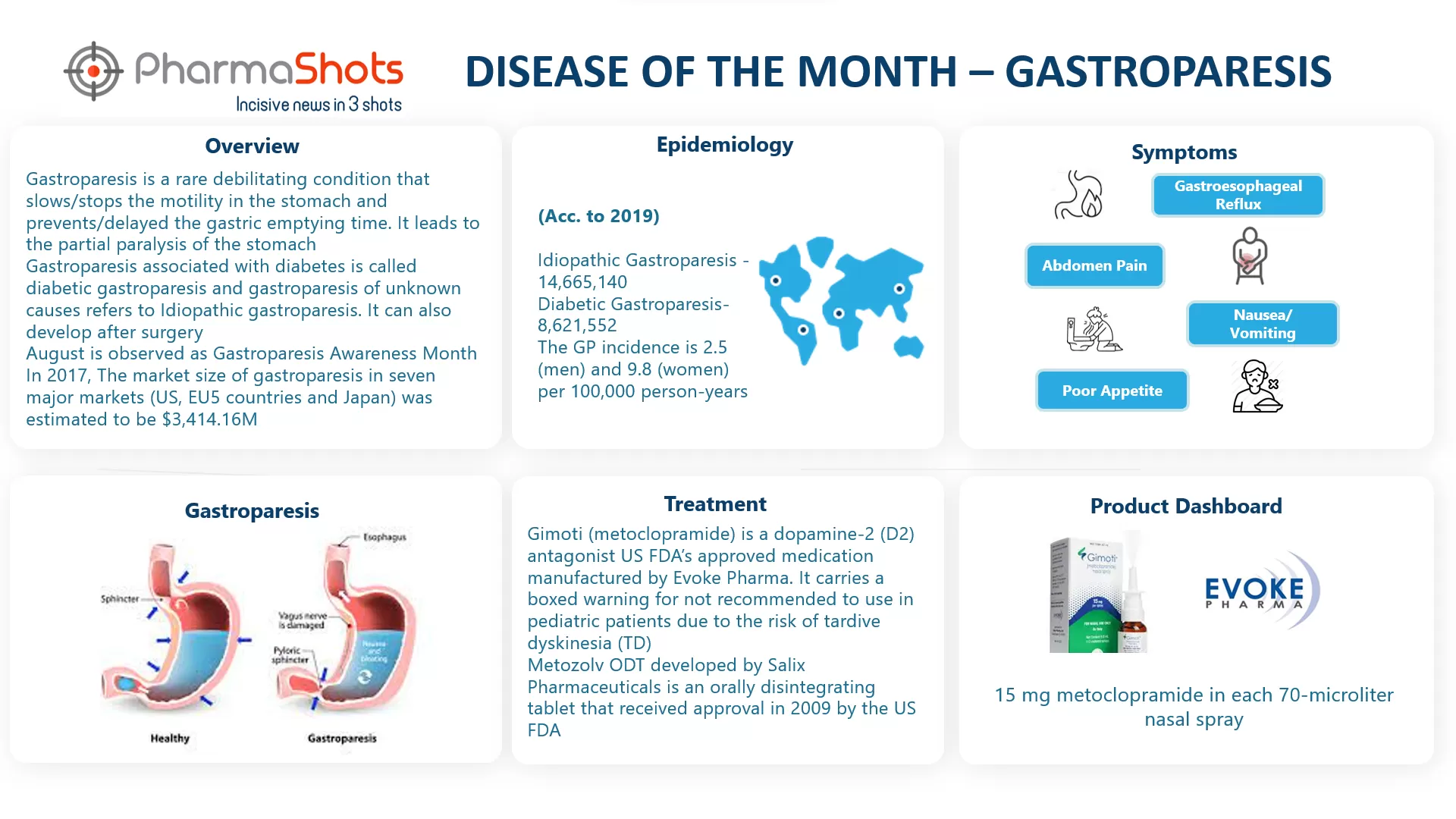
Evarsana Signs an Agreement with Evoke Pharma to Commercialize Gimoti in the US
Shots:
- Evoke to receive $5M revolving credit facility following FDA approval of Gimoti while Eversana will utilize its outsourced services to commercialize and distribute Gimoti in the US
- Evoke will remain responsible for legal- regulatory- and manufacturing activities and retain 80% of the profit and record sales for Gimoti to Eversana’s third-party logistics division. Evarsana to receive reimbursement of cost & % of product’s profits in the mid to high teens when Gimoti net sales outstrip its development & commercialization costs
- The term of the agreement is 5yrs. following FDA approval- after which Evoke will recapture all commercialization activities while both companies can terminate the agreement on certain events. Gimoti is a nasal spray- designed to provide relief to women with acute and recurrent diabetic gastroparesis with its anticipated PDUFA sate as 19 Jun- 2020
Click here to read full press release/ article | Ref: Evoke Pharma | Image: Evoke Pharma

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com