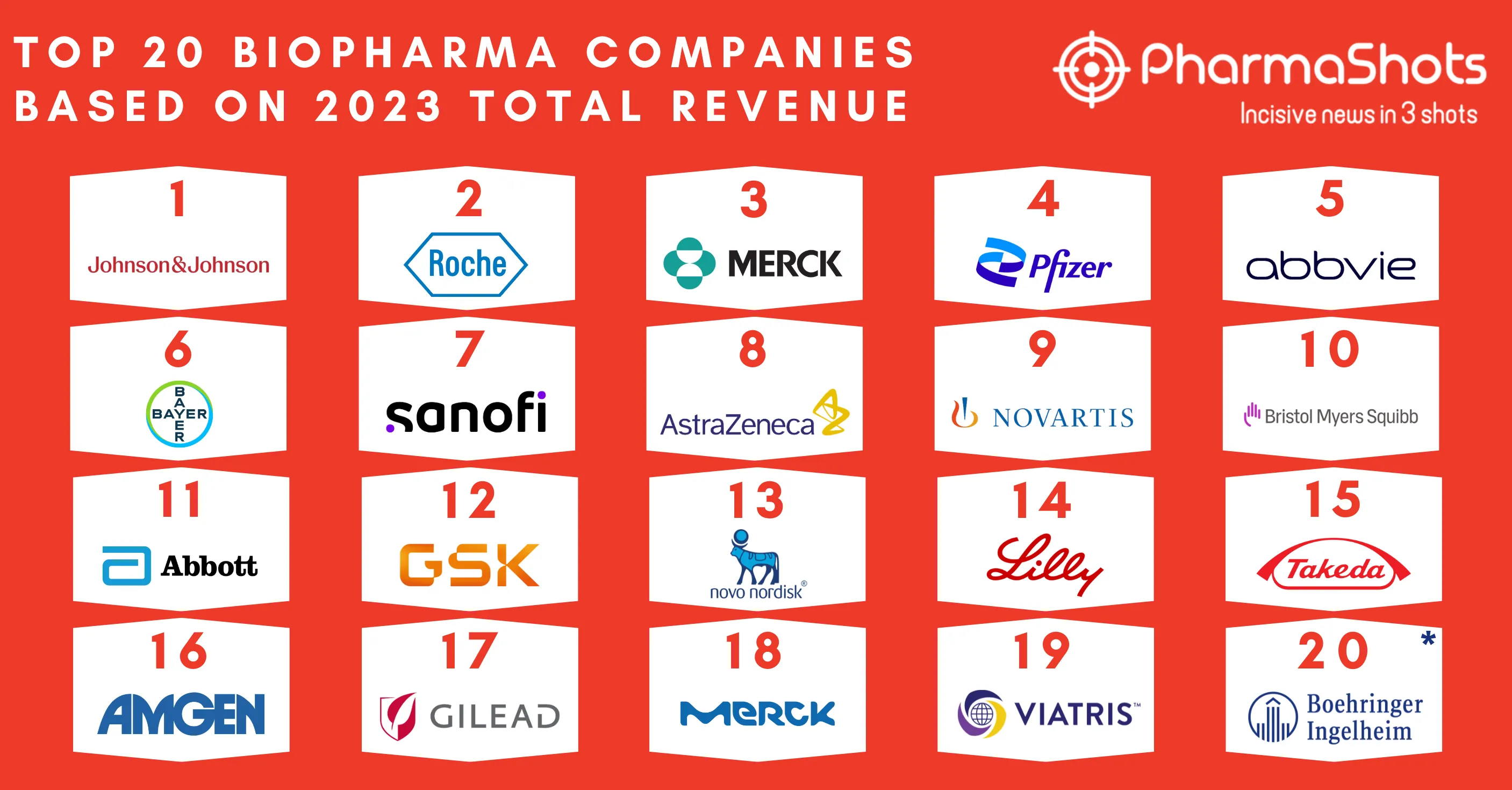
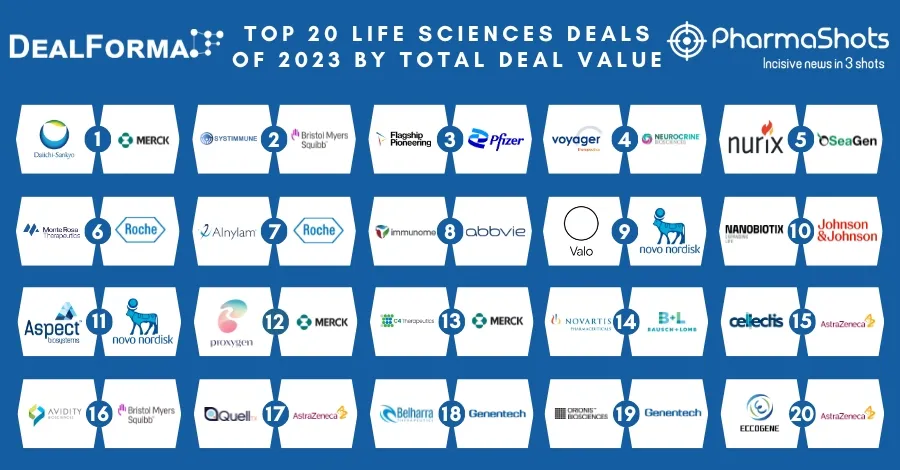
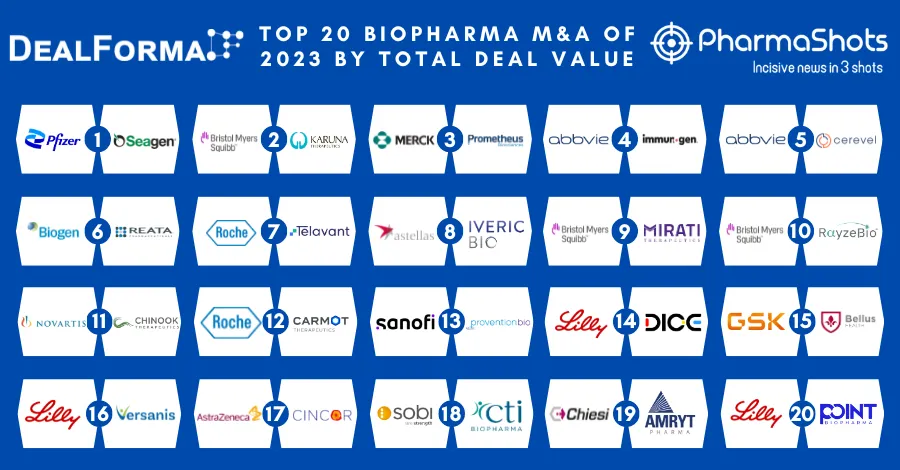
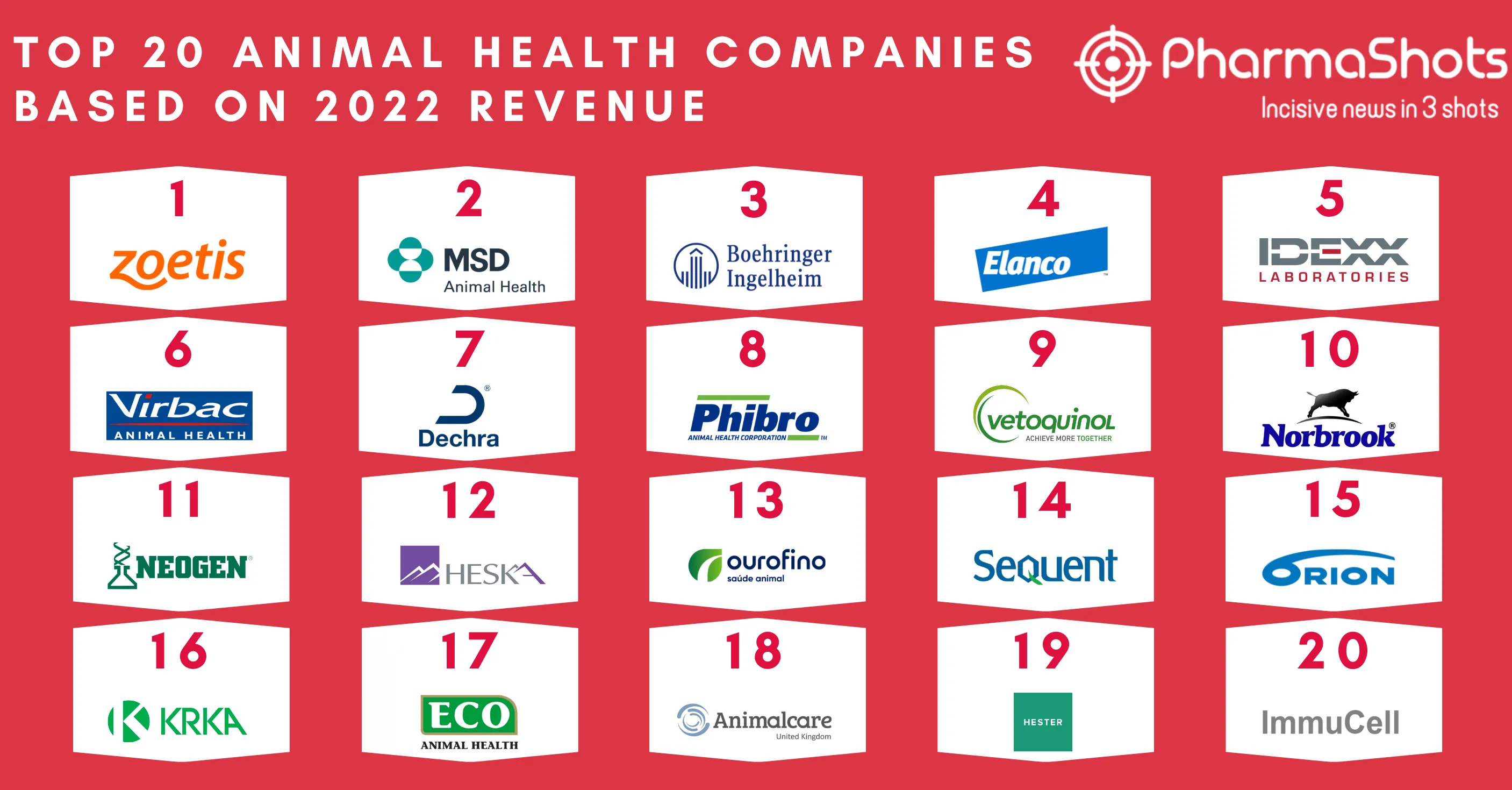
Merck KGaA and Pfizer's Bavencio (avelumab) + Inlyta (axitinib) Receive FDA's Approval as 1L Treatment for Advanced Renal Cell Carcinoma
Shots:
- The approval is based on P-III JAVELIN Renal 101 study (NCT02684006) results assessing bavencio (avelumab) + Inlyta (axitinib) vs sunitinib in 886 patients in a ratio (1:1) with untreated advanced RCC regardless of tumor PD-L1 expression
- The P-III JAVELIN Renal 101 study results: improvement in PFS (13.8 vs 8.4mos.); ORR (51.4% vs 25.7%); published in The New England Journal of Medicine
- Bavencio (avelumab) is a PD-L1 antibody- developed under the co-development and co-commercialization deal b/w Merck KGaA & Pfizer in 2014 & Inlyta is a tyrosine kinase inhibitor indicated for advanced RCC. The combination is under regulatory review in EU & Japan
Ref: Pfizer | Image: Pinterest
Click here to read the full press release

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com